All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Phase I CheckMate 039: nivolumab plus ipilimumab for R/R HL, NHL and MM
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology (ASH) took place in San Diego, CA, on 3–6 December.
On Saturday 3rd December, an oral abstract session was held between 2:00pm and 3:30pm in the “Novel Therapeutics and the integration of PET Scans in Hodgkin Lymphoma and Peripheral T-Cell Lymphomas” category. This session was moderated by Alison J. Moskowitz, MD, and Steven M. Horwitz, MD, from the Memorial Sloan Kettering Cancer Center.
Abstract #183 was presented during this session, titled “A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039)” by Stephen Ansell, MD PhD, of the Mayo Clinic, Rochester, MN, and colleagues.
Presented during this session was results of a cohort study of the CheckMate 039 (NCT01592370) phase 1b study, which evaluated the efficacy and safety of combining nivolumab and ipilimumab in patients with HL, B-NHL (FL and DLBCL), T-NHL (CTCL and PTCL), and MM. Patients must have R/R disease after ≥2 previous lines of treatment and adequate organ function. Safety was the primary endpoint and secondary endpoints included investigator-assessed ORR, best Overall Response, PFS, and DoR.
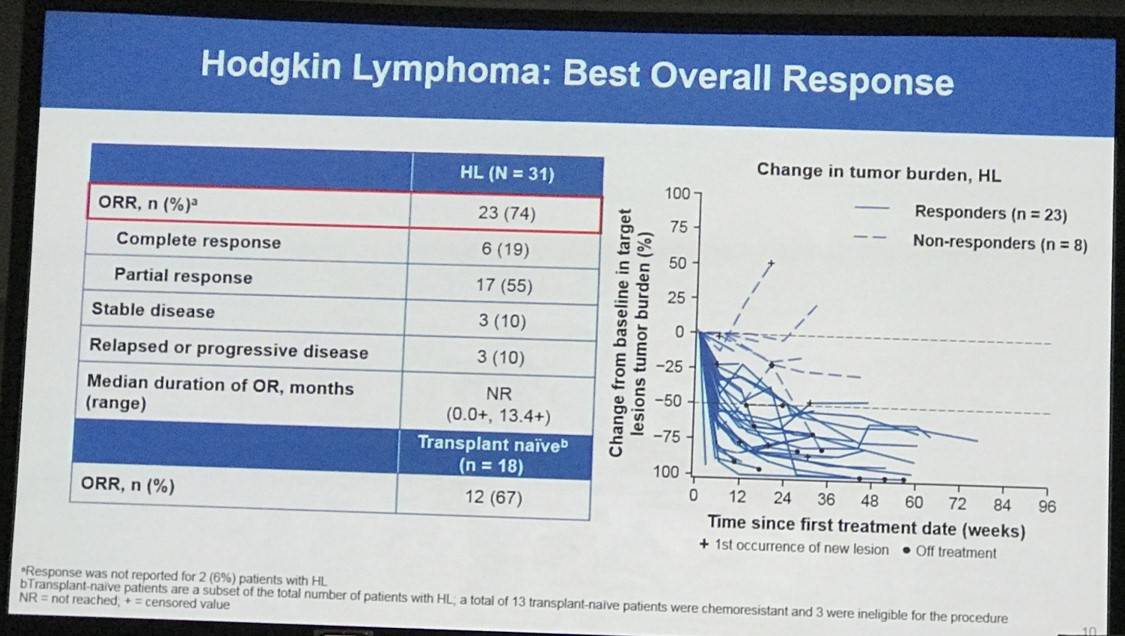
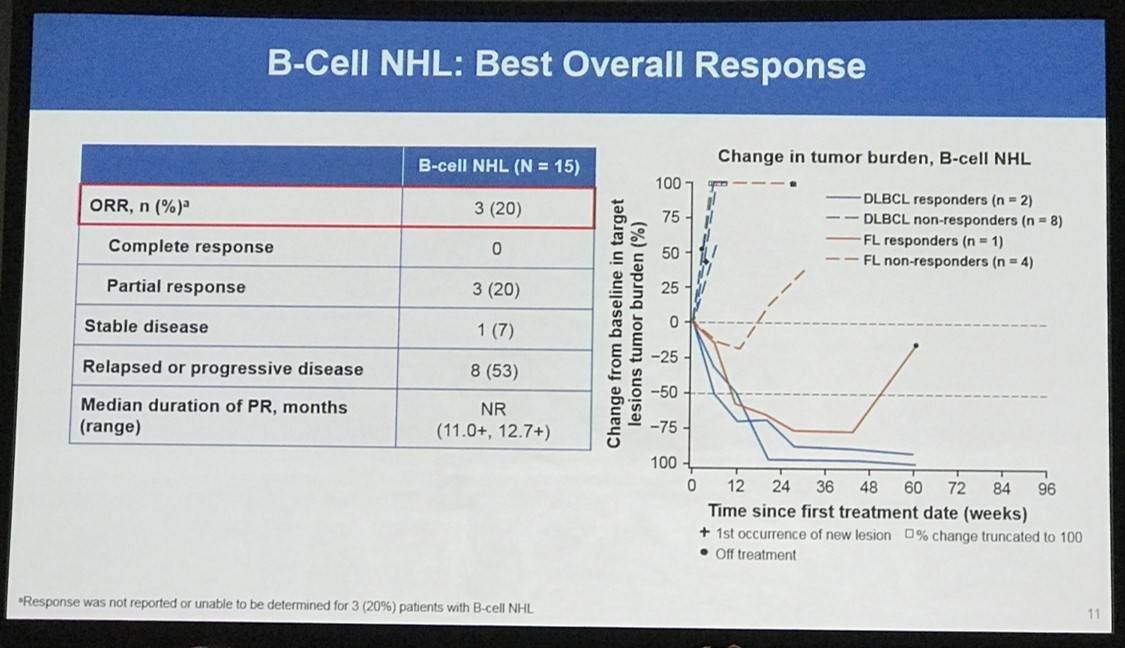
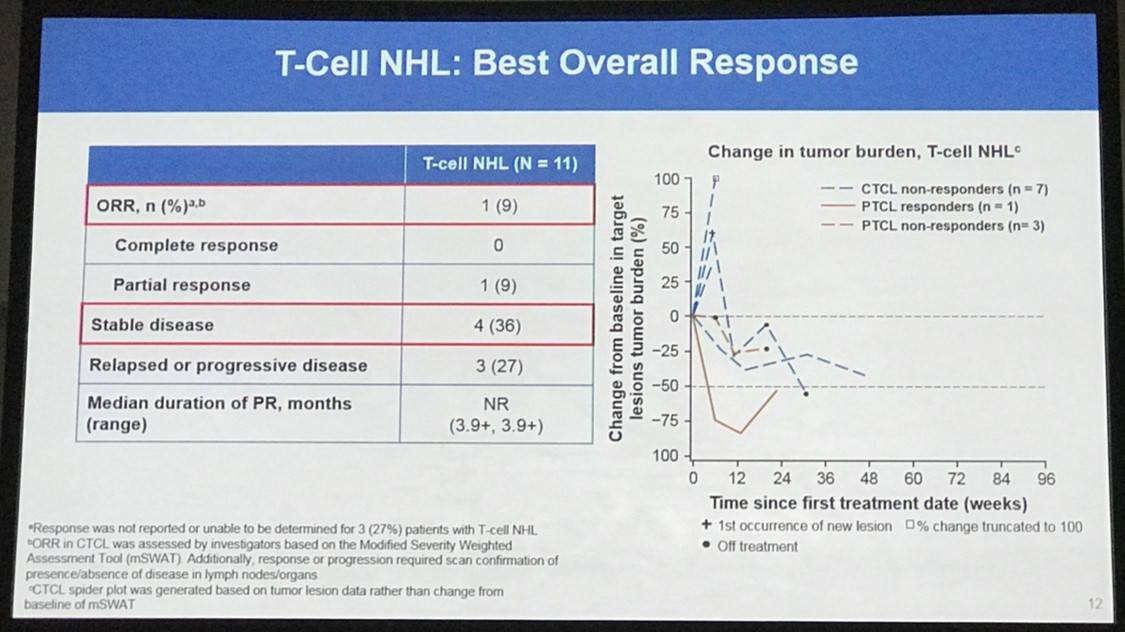
Overall, 65 patients were treated: 31 with HL, 15 with B-NHL, 11 with T-NHL, 7 with MM, and 1 with PMBL who was only included in the overall safety cohort. Across all cohorts, median follow-up duration was 11.4 months:
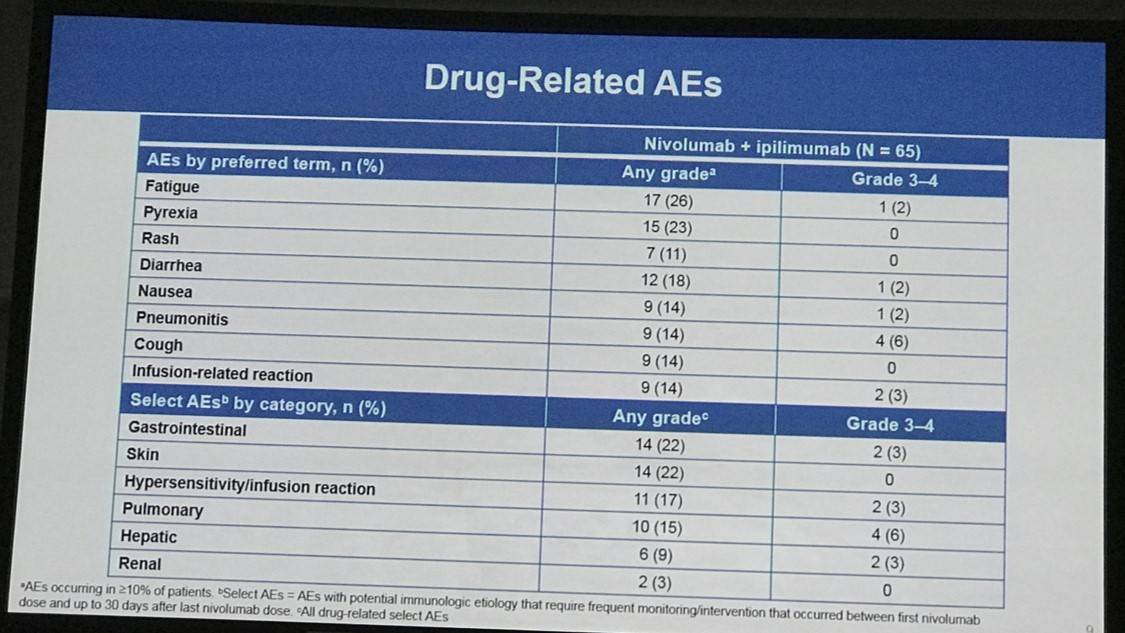
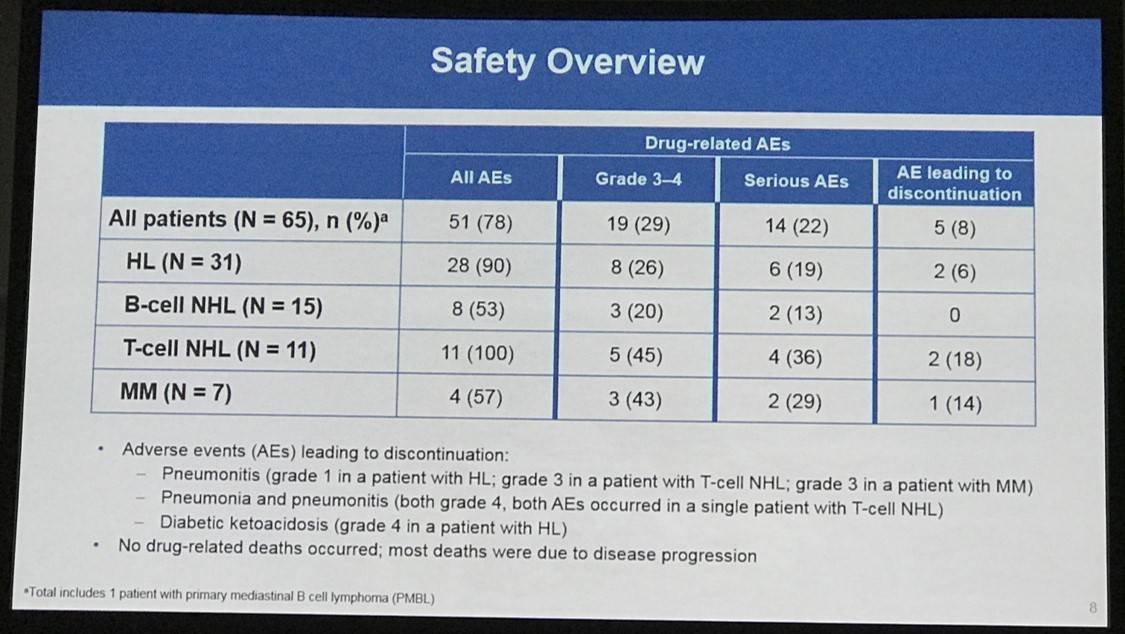
- Treatment discontinuation was reported in 5 patients (8%) due to a drug-related AE
- The most frequently reported drug-related AEs of any grade were fatigue (26%; 17 patients), pyrexia (23%; 15 patients), and diarrhea (18%, 12 patients)
- Drug-related ≥grade 3 AEs occurred in 19 patients (29%) and SAEs occurred in 31 patients (48%)
- Disease progression resulted in the death of 22 (34%) patients (2 HL patients, 10 B-NHL patients, 5 T-NHL patients, 4 MM patients, and 1 PMBL patient)
This abstract presentation was concluded by stating that nivolumab plus ipilimumab in this cohort of heavily pretreated patients showed an efficacy and safety profile similar to that documented in the past for nivolumab monotherapy for patients with HL, NHL, and MM. The role of ipilimumab in this group of patients may become clearer with longer follow-up.
Abstract
Introduction: Nivolumab (nivo) is a fully human IgG4 monoclonal antibody (mAb) targeting programmed death receptor-1 (PD-1). Nivo has demonstrated clinical activity and an acceptable safety profile in a phase 1b study (NCT01592370; CheckMate 039) in patients (pts) with relapsed/refractory hematologic malignancies. In pts diagnosed with Hodgkin lymphoma (HL), after a median 86 weeks of follow-up, 7/20 responders maintained a response for >1.5 years (Ansell S et al. Blood 2015;126:583), and after a median follow-up of 67 weeks, clinical activity (investigator-assessed objective response rate) was demonstrated in follicular lymphoma (FL; 40%), diffuse large B-cell lymphoma (DLBCL; 36%), mycosis fungoides (15%), and peripheral T-cell lymphoma (PTCL; 40%) (Lesokhin AM et al. J Clin Oncol 2016;34:2698). CheckMate 039 also included a cohort of pts who had received nivo in combination with ipilimumab (ipi), a fully human mAb targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4). Combination of CTLA-4 and PD-1 blockade has shown superior efficacy compared with nivo or ipi alone in preclinical studies and solid tumor malignancies (Wolchok JD et al. N Engl J Med 2013;369:122; Larkin JM et al. N Engl J Med 2015;373:22; Antonia SJ et al. Lancet Oncol 2016;17:883). The aim of this cohort study was to evaluate the safety and efficacy of combined immune checkpoint blockade (nivo+ipi) in pts with the following hematologic malignancies: HL, B-cell non-Hodgkin lymphoma (B-NHL; FL and DLBCL), T-cell NHL (T-NHL; cutaneous T-cell lymphoma [CTCL] and PTCL]), and multiple myeloma (MM).
Methods: Nivo+ipi were given at 3 mg/kg IV and 1 mg/kg IV, respectively, every 3 weeks for 4 doses, followed by nivo monotherapy (3 mg/kg) every 2 weeks for up to 2 years. Pts with any of the above histologies, relapsed or refractory disease after ≥2 prior lines of therapy, and adequate organ function were included in the study. Prior systemic therapy may have included chemotherapy and autologous hematopoietic stem cell transplantation (auto-HSCT). Prior anti–PD-1 therapy and allogeneic (allo)-HSCT were not permitted. The primary endpoint was safety. Secondary endpoints included investigator-assessed objective response rate (ORR), best overall response, duration of response (DOR), and progression-free survival (PFS).
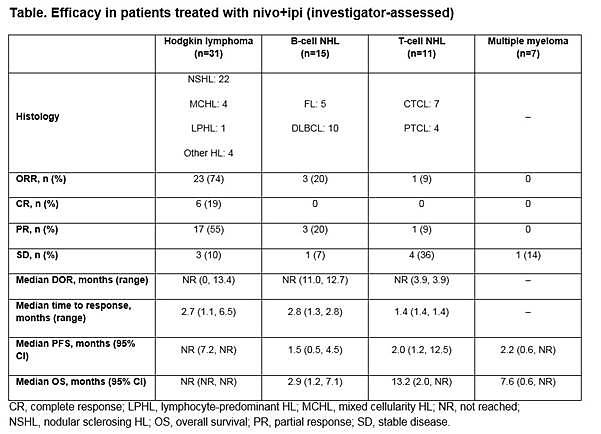
Results: In total, 65 pts were treated with nivo+ipi (31 HL, 15 B-NHL, 11 T-NHL, 7 MM, and 1 with primary mediastinal B-cell lymphoma [PMBL] who was included in the overall safety cohort only). Median (range) number of prior systemic therapies was 4 (2, 10; HL), 3, (1, 16; B-NHL), 4 (1, 11; T-NHL), and 5 (2, 20; MM). Among patients with HL, only 13% (4/31) had prior auto-HSCT. 2 pts with HL and 1 with T-NHL proceeded to allo-HSCT after stopping study therapy. Across all cohorts, median follow-up was 11.4 months. 5 pts (8%) discontinued due to a drug-related adverse event (AE). The most common drug-related AEs of any grade were fatigue (17 pts [26%]), pyrexia (15 [23%]), and diarrhea (12 [18%]). 19 pts (29%) had a drug-related AE of grade ≥3. 31 pts (48%) had a serious AE. 24 pts (37%) died: HL 2 pts, B-NHL 11, T-NHL 6, MM 4, PMBL 1. Among those pts, 22 (34%) were from disease progression (HL 2 pts, B-NHL 10, T-NHL 5, MM 4, PMBL 1); no deaths were due to an AE. Clinical outcome data are presented (Table).
Conclusions: These are the first reported data of combination checkpoint blockade therapy in hematologic malignancies. Overall, the combination of nivo+ipi in these heavily pretreated patients demonstrated a safety and efficacy profile similar to that previously reported for nivo monotherapy in HL, NHL, and MM. Additional follow-up may further clarify the role of ipi in this cohort of patients. In this predominantly transplant-naïve group of patients with HL, the efficacy of nivo+ipi was similar to that seen in patients with relapsed/refractory HL treated with nivo alone.
- Ansell S. et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039). Oral Abstract #183: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








