All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Lenalidomide maintenance in R/R DLBCL
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, on December 3–6, 2016. On Sunday 4th December, an oral abstract session was held between 4:30pm and 6.00pm in the “Aggressive Lymphoma (Diffuse Large B-Cell and other aggressive B-Cell Non-Hodgkin Lymphomas) – results from prospective clinical trials: Novel Approaches in Aggressive Lymphoma” category. This session was moderated by Jason R. Westin, MD MS, from The University of Texas MD Anderson Cancer Center, and Laurie H Sehn, MD, of the British Columbia Cancer Agency.
Abstract #474 was the final abstract presented during this session, titled “Lenalidomide maintenance significantly improves survival figures in patients with relapsed Diffuse Large B- Cell Lymphoma (rDLBCL) who are not eligible for Autologous Stem Cell Transplantation (ASCT): Final results of a multicenter phase II trial” by Andrés J.M. Ferreri from the IRCCS San Raffaele Scientific Institute, Italy, and colleagues.
Andrés J.M. Ferreri and colleagues are conducting a multicenter phase II trial (NCT00799513), which explores the safety and efficacy of lenalidomide maintenance in patients with chemosensitive rDLBCL who are not eligible for ASCT or are experiencing relapse after ASCT. 1-year Progression Free Survival (PFS) was the primary endpoint of this study. The trial used a Simon’s two stage optimal design to demonstrate 1-year PFS. Forty-six patients, median age 72 years (34–86), de novo DLBCL (36 patients), transformed DLBCL (10 patients), were included in the trial.
- Lenalidomide was well tolerated and AEs were uncommon
- Due to neutropenia (n = 12), rash (n = 7), diarrhea (n = 2), and neurotoxicity (n = 2), 23 patients had lenalidomide dose reduction and 6 had discontinuation
- At 56 months follow up, death occurred due to acute toxicity (n = 1) and secondary Myelodysplastic Syndrome (n = 1)
- Twenty-eight patients were progression free after 1-year, significantly higher than the threshold (n ≥ 19)
- 21 events occurred during the observation period; progressive disease (n = 19), death due to toxicity (n = 1) and death while off therapy (n = 1)
- Primary endpoint, 1-year PFS was 70%
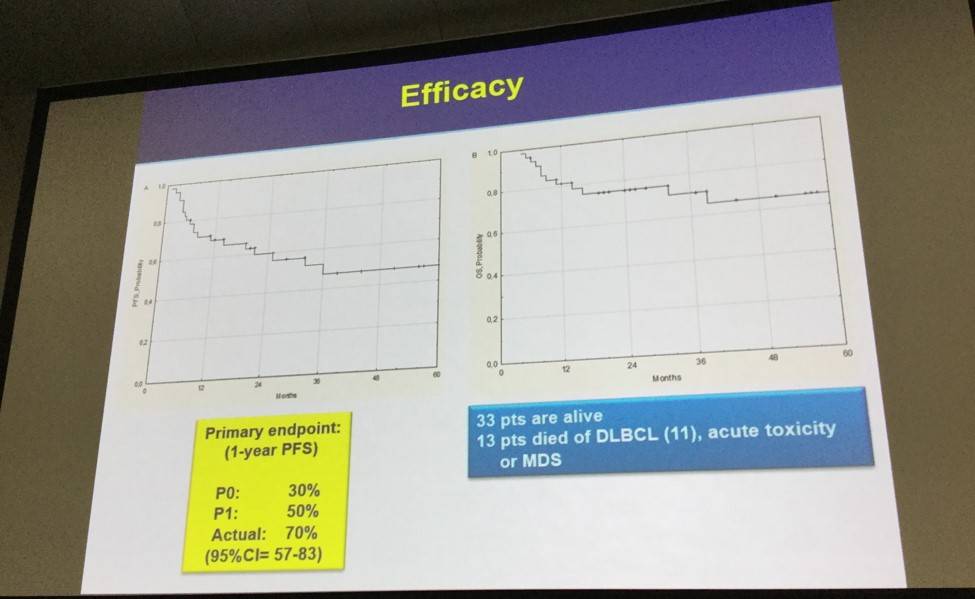
- Fifty-nine percent of patients had longer responses to lenalidomide versus response duration after prior therapy
- 1-year PFS was 64 ± 11% GCB-DLBCL, 67 ± 11% non-GDB-DLBCL (P = 0.67)
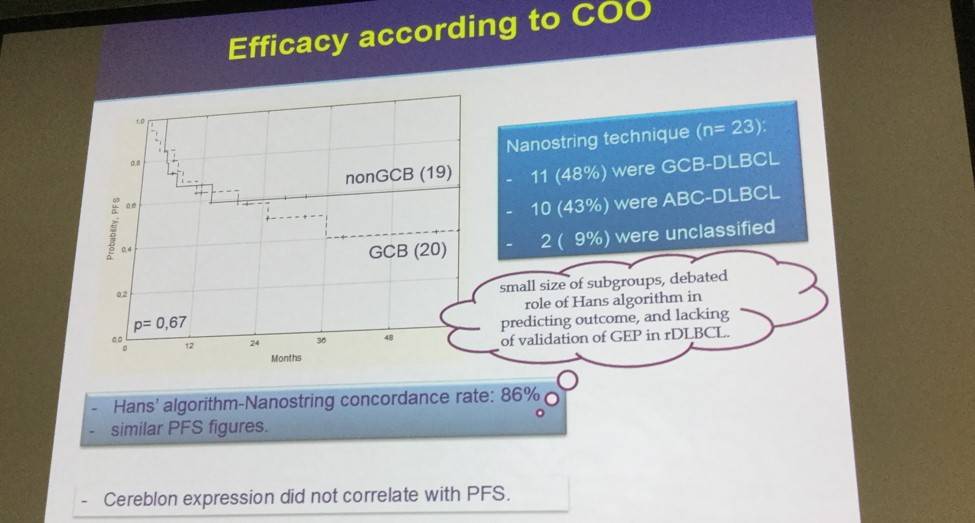
- No significant association between PFS and cereblon expression
- Thirty-three patients are alive with a 1- and 3-year Overall Survival (OS) of 81 ± 6% and 71 ± 8%, respectively
The presentation was concluded by highlighting that the results obtained from this trial promotes the use of lenalidomide maintenance in patients with chemosensitive relapse of DLBCL not eligible for ASCT or experiencing relapse after ASCT. There were survival benefits for patients with de novo or transformed DLBCL and also in patients with either GCB- or non-GCB DLBCL and lenalidomide was well tolerated in the elderly population. Further investigations are warranted for the use of immunomodulatory drugs as maintenance in high-risk patients.

Abstract
Background: Patients (pts) with rDLBCL not eligible for ASCT or experiencing relapse after ASCT have a low likelihood of cure. Single-drug maintenance after salvage therapy may be an attractive strategy to prolong survival in these pts. Lenalidomide (LEN) is a suitable candidate for long-lasting maintenance as it is an oral drug, active against DLBCL that can be taken for years with an acceptable toxicity profile. Accordingly, we designed a multicentre phase II trial addressing LEN maintenance in pts with chemosensitive relapse of DLBCL not eligible for ASCT or experiencing relapse after ASCT (clinicaltrials.gov NCT00799513).
Methods: HIV-neg pts (age ≥18 ys) with histologically-proven de novo or transformed DLBCL and relapsed disease responsive to conventional rituximab-containing salvage therapy were registered and treated with LEN 25 mg/day for 21 days out of 28, until lymphoma progression or unacceptable toxicity. Primary endpoint was 1-year progression-free survival (PFS). Simon's two-stage optimal design was used. To demonstrate a 1-yr PFS improvement from 30% (P0) to 50% (P1), 47 pts (one-sided; α 5%; β 80%) were needed. Maintenance would be considered effective if ≥19 pts were progression-free survivors at 1 yr. Cell of origin was assessed by NanoString Technology and Hans algorithm, and cereblon expression was assessed by immunohistochemistry.
Results: 46 of 48 enrolled pts were assessable (median age 72 ys; range 34-86); 36 pts had de novo DLBCL, 10 had transformed DLBCL. All pts were previously treated with anthracycline- and rituximab-based combination, plus ASCT in 6 pts; the median TTP after the prior therapy was 16 months (range 3-121). Thirty-three pts were enrolled at 1st relapse, 13 at 2nd relapse; salvage therapy contained high doses of cytarabine or ifosfamide in two-thirds of cases, and response was complete in 26 pts and partial in 20. Most pts had unfavourable features: IPI ≥2 in 38 (83%) pts, advanced stage in 35 (76%), extranodal disease in 29 (63%), high LDH level in 21 (46%); 28 (61%) pts were older than 70 ys.
At a median follow-up of 25 (range 6-87) months, 556 LEN courses were delivered, with an average of 12 courses/pt (range 3-41); 19 pts are still in treatment. LEN was well tolerated: with the exception of neutropenia, grade 3-4 toxicities were uncommon, occurring in ≤3% of delivered courses. Infections were rare, and well controlled with oral antibiotics (grade 1-2 in 8 courses; grade 3 in 3). LEN dose reduction was indicated in 23 pts (transient in 19), and was due to neutropenia (12), rash (7), diarrhoea (2), and neurotoxicity (2); LEN was discontinued in 6 of them. One (2%) pt died of acute toxicity (intestinal infarction) and one due to secondary myelodysplastic syndrome at 56 months of follow-up. Pts with HBV/HCV seropositivity (n=12) or prior ASCT (n=6) did not experience unexpected toxicity after >1 yr of treatment.
At one year from trial registration, 28 pts were still progression free, which was significantly higher than the pre-determined efficacy threshold (n≥19). During the whole observation period, there were 21 events: progressive disease in 19 pts, death of toxicity in one, death while off therapy in one, with a 1-yr PFS (primary endpoint) of 70 ± 7%. The duration of response to LEN was longer than response duration after the prior treatment line in 27 (59%) pts, and was twice as long in 15 of them.
The benefit of LEN maintenance was observed both in pts with de novo or transformed DLBCL. According to the Hans’ algorithm, the 1-yr PFS was 64 ± 11% for GCB-DLBCL and 67 ± 11% for nonGCB-DLBCL (p= 0.67). Results using the Nanostring technique were consistent with the Hans’ algorithm, with a concordance rate of 86%. There was no significant association between cereblon expression and PFS. Multivariate analysis confirmed that treatment at first relapse and a prior TTP ≥12 months were independently associated with better PFS. Overall, 33 (72%) pts are alive, with a 1- and 3-yr OS of 81 ± 6% and 71 ± 8%, respectively.
Conclusions: With the limitations of a non-randomized design, this trial soundly promotes the use of LEN maintenance in pts with chemosensitive relapse of DLBCL not eligible for ASCT or experiencing relapse after ASCT. LEN was well tolerated in this elderly population, with survival benefit both in pts with de novo or transformed DLBCL, and both in pts with GCB- or nonGCB-DLBCL. These results warrant further investigation of immunomodulatory drugs as maintenance in these high-risk pts.
- Ferreri A.J.M. et al. Lenalidomide maintenance significantly improves survival figures in patients with relapsed Diffuse Large B- Cell Lymphoma (rDLBCL) who are not eligible for Autologous Stem Cell Transplantation (ASCT): Final results of a multicenter Phase II trial. 2016 December 4; Oral Abstract #474: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








