All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Long Term Follow-up of SWOG S0016 Phase III Randomized Study Continued Excellent Outcomes in Previously Untreated Follicular Lymphoma Patients after Treatment with CHOP Plus Rituximab or CHOP Plus (131) Iodine-Tositumomab -
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, and on December 5th Mazyar Shadman, MD, MPH, from the Division of Medical Oncology, University of Washington, Seattle, presented a long-term follow-up update to the SWOG S0016 randomized phase-III study aimed at comparing the efficacy of R-CHOP with CHOP-RIT (with 131 iodine-tositumumuab) in untreated FL patients.
Highlights:
- 531 pts randomized to receive either:
- R-CHOP (n=264)= 6 cycles of CHOP every 3 weeks with 6 doses of rituximab
- CHOP-RIT (n=267)= 6 cycles of CHOP every 3 weeks with consolidative RIT with 131 iodine-tositumumuab
- Both groups had balanced baseline characteristics
- CR: R-CHOP=40%, CHOP-RIT=45%
- Median follow-up 9.6 years (0.1-14.4 years)
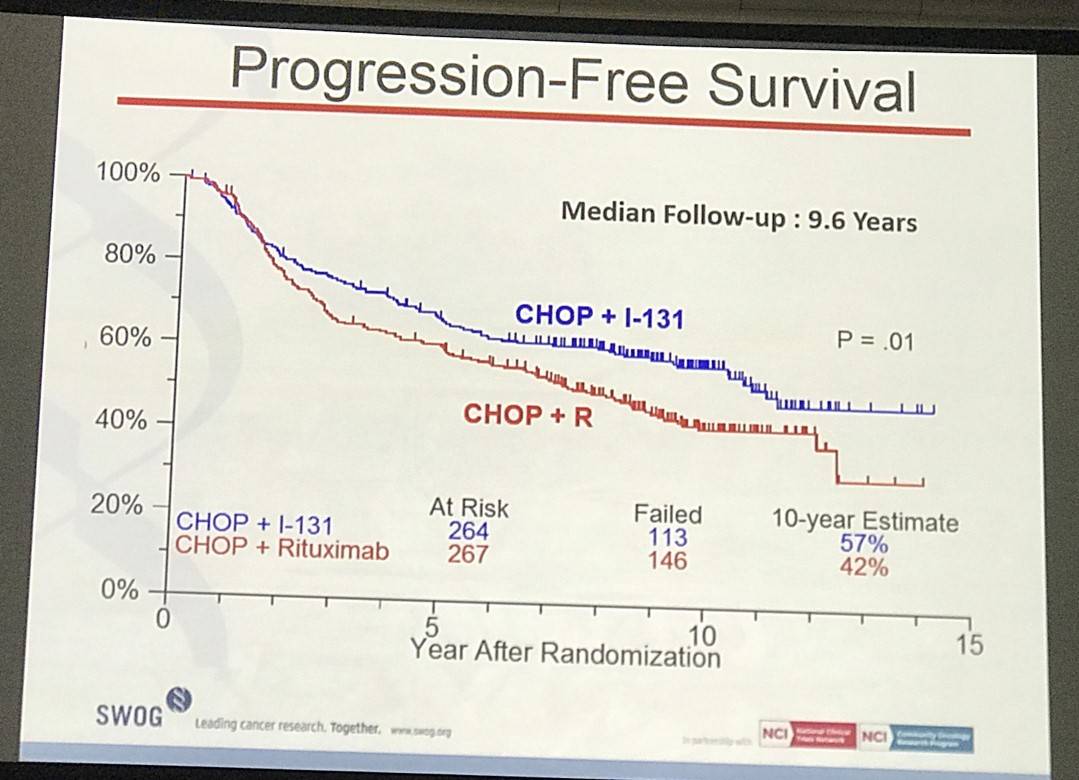
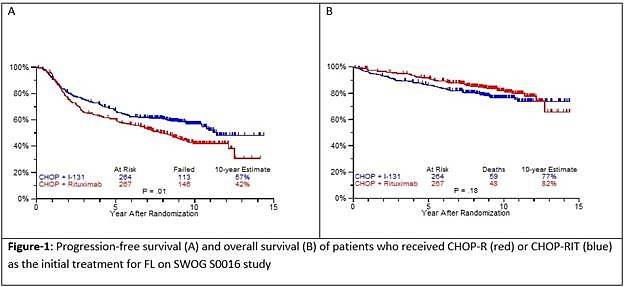
- Estimated 10yr PFS R-CHOP=42%, CHOP-RIT=57% (P=0.01)
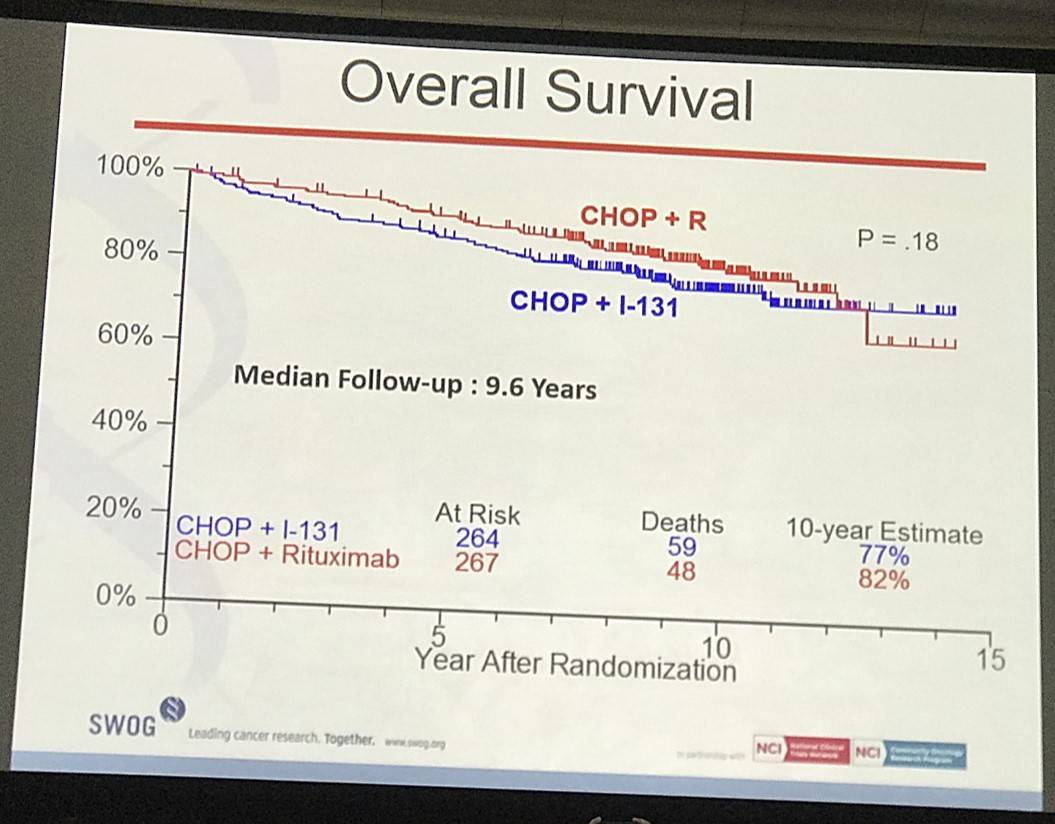
- Estimated 10yr OS R-CHOP=82%, CHOP-RIT=77% (P=0.18)
- 2yr relapse-free survival R-CHOP=76%, CHOP-RIT=81% (P=0.015)
Mazyar Shadman concluded by stating that FL patients treated with either CHOP-RIT or R-CHOP both showed ‘outstanding survival’ after 10 years. While CHOP-RIT was shown, with this protocol, to have a longer PFS than R-CHOP, the 10 year OS was similar illustrating how important it is to perform long-term studies when comparing treatment options.
Abstract:
Background: Southwest Oncology Group (SWOG) S0016 was a phase III randomized study that compared safety and efficacy of cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab (CHOP-R) with CHOP followed by consolidation with (131) iodine-tositumomab radioimmunotherapy (CHOP-RIT) for previously untreated follicular lymphoma (FL) patients. As reported previously, the study demonstrated excellent outcomes in both arms with 2-year progression-free survival (PFS) of 76% and 80% and 2-year overall-survival (OS) of 97% and 93% in the CHOP-R and CHOP-RIT arms, respectively. (Press. et al. JCO, 2012) We now report the long term outcome of patients who were treated on the S0016 study.
Methods: Between 2001 and 2008, 531 previously untreated advanced follicular lymphoma patients were randomized to receive either 6 cycles of CHOP every 3 weeks with 6 doses of rituximab (CHOP-R) or 6 cycles of CHOP every 3 weeks followed by RIT. Patients with advanced-stage disease (bulky stage II, III or IV) with any pathologic grade (1, 2 or 3) were eligible.
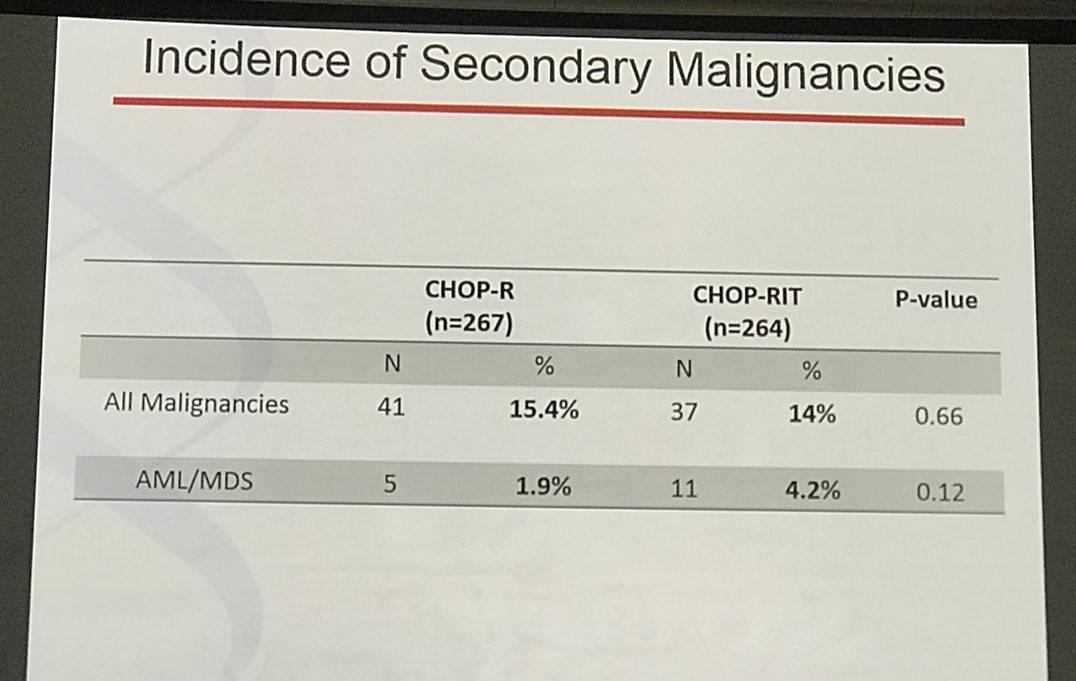
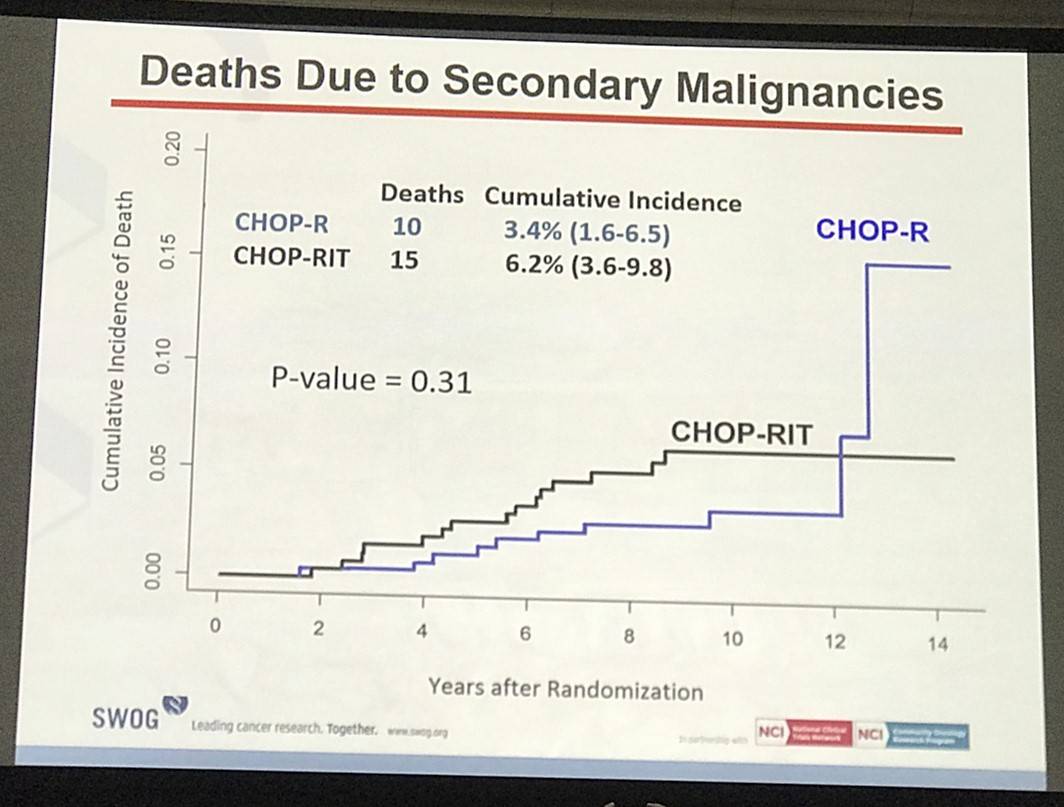
Results: Patients on CHOP-R (n=264) and CHOP-RIT (n=267) arms had balanced baseline characteristics including age (median 54.5 vs. 53.4 years), presence of B symptoms (29% vs. 26%), pathologic grade 3 (8% vs. 9%), “high” FLIPI risk group (22% vs. 26%), stage IV disease (59% vs. 63%), elevated β2M (53% vs. 55%), bone marrow involvement (56% vs. 55%) and bulky disease (24% vs. 26%). Objective remissions were documented in 226 of 267 eligible patients treated with CHOP-R (85%) and in 226 of 264 patients with CHOP-RIT (86%). Complete remission (CR) rate was 40% in the CHOP-R and 45% in the CHOP-RIT arm. Neutropenia was the most common adverse event (AE) in both arms (48% vs. 51%). Rate of grade 3-5 AEs were not different except for thrombocytopenia which was more common in the CHOP-RIT group (18% vs. 2%). After a median follow-up of 9.6 years (range 0.1 – 14.4 years), the estimated 10-year PFS was 42% (95% CI: 35.6%, 48.5%) in the CHOP-R arm and 57% (95% CI: 50.5%, 63.2%) in the CHOP-RIT arm (p-value = 0.01). 51% of responders on the CHOP-R arm and 38% of responders on the CHOP-RIT arm have relapsed (p-value= 0.01). The estimated 2-year relapse-free survival was 76% for CHOP-R patients and 81% for CHOP-RIT patients (p-value=0.15). There were 48 deaths in the CHOP+R arm and 59 deaths in the CHOP-RIT arm with an estimated 10-year OS of 82% (95% CI: 76.3%, 86.4%) with CHOP-R and 77% (95% CI: 70.5%, 81.4%) with CHOP-RIT (p-value = 0.18). (Figure-1) During the follow-up, 41 patients (15.4%) in the CHOP-R arm and 37 patients (14%) in the CHOP-RIT arm developed secondary malignancies. MDS/AML were more common in the CHOP-RIT arm (11 patients; 4.2%) compared to CHOP-R arm (5 patients; 1.9%) with a relative risk of 2.2 (95% CI: 0.8-6.3) (P-value 0.12).
Conclusion: With almost 10 years of follow-up, patients with FL treated on protocol S0016 show outstanding survival in both CHOP-R and CHOP-RIT arms, and represent the best published long-term outcomes to date in this disease. Indeed, almost half of patients enrolled still remain progression-free, confirming registry experiences. While CHOP-RIT provided significantly longer PFS and lower relapse rate compared to CHOP-R in our study, the OS rate was not different between 2 arms due in part to higher incidence of fatal MDS/AML in the CHOP-RIT group, emphasizing the importance of long-term follow-up, and calling into question the validity of PFS as a surrogate for OS in this setting. Given these outstanding outcomes, we believe chemoimmunotherapy should remain the standard induction approach for patients with high-risk FL until long-term follow-up of alternative approaches demonstrates superiority.
Support: NIH/NCI grants CA180888, CA180819, CA180821 and in part by GlaxoSmithKline
- Shadman M. et al. Continued Excellent Outcomes in Previously Untreated Follicular Lymphoma Patients after Treatment with CHOP Plus Rituximab or CHOP Plus (131) Iodine-Tositumomab - Long Term Follow-up of Phase III Randomized Study SWOG S0016. 2016 December 5; Oral Abstract #616: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








