All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
iwCLL 2017 | Clinical and biological insights into Monoclonal B-Lymphocytosis
Bookmark this article
On Monday 13th May, the first session during iwCLL 2017 was titled “Unraveling the factors leading to the development of CLL”, and was chaired by Daniel Catovsky (Institute of Cancer Research, London, UK) and Stephan Stilgenbauer (University of Ulm, Germany).
The first talk of the session, titled “Monoclonal B-Lymphocytosis: Recent Clinical and Biologic Insights”, was presented by Tait Shanafelt from the Mayo Clinic.
History, Classification, and Progression
In the 1990s, the CDC carried out environmental health studies which found that 0.6% (9/1,499) of people aged 45 years or older had a monoclonal B-cell population by 2 color flow cytometry and the prevalence of this clone was several hundred times more common than CLL/SLL (Vogt et al. 2007).
Further studies have been carried out using more sensitive flow cytometry techniques to reveal the clone in approximately 3–5% of cases aged over 40 years, 5–10% of cases aged over 60 years, and potentially 75% of cases aged over 90 years.
Shanafelt then outlined the diagnostic criteria (Marti et al. 2005) for CLL-Like Monoclonal B-Lymphocytosis (MBL):
- Circulating population of clonal B-cells
- Total B-cell count <5x109/L
- No other features of lymphoproliferative disorder
- Phenotype in majority is that of CLL
Moreover, Shanafelt outlined the differences between Low Count (LC) and High Count (HC)-MBL:
- LC-MBL: 95% of patients in population screening have clonal B-cell count <60/µl; CLL phenotype makes up <10% of total B-cells (can be oligoclonal or even polyclonal)
- HC-MBL: 95% of patients in clinical practice have clonal B-cell count >450/µl; CLL phenotype makes up majority of B-cells (a higher clonal B-cell proportion does not correlate with risk of progression)
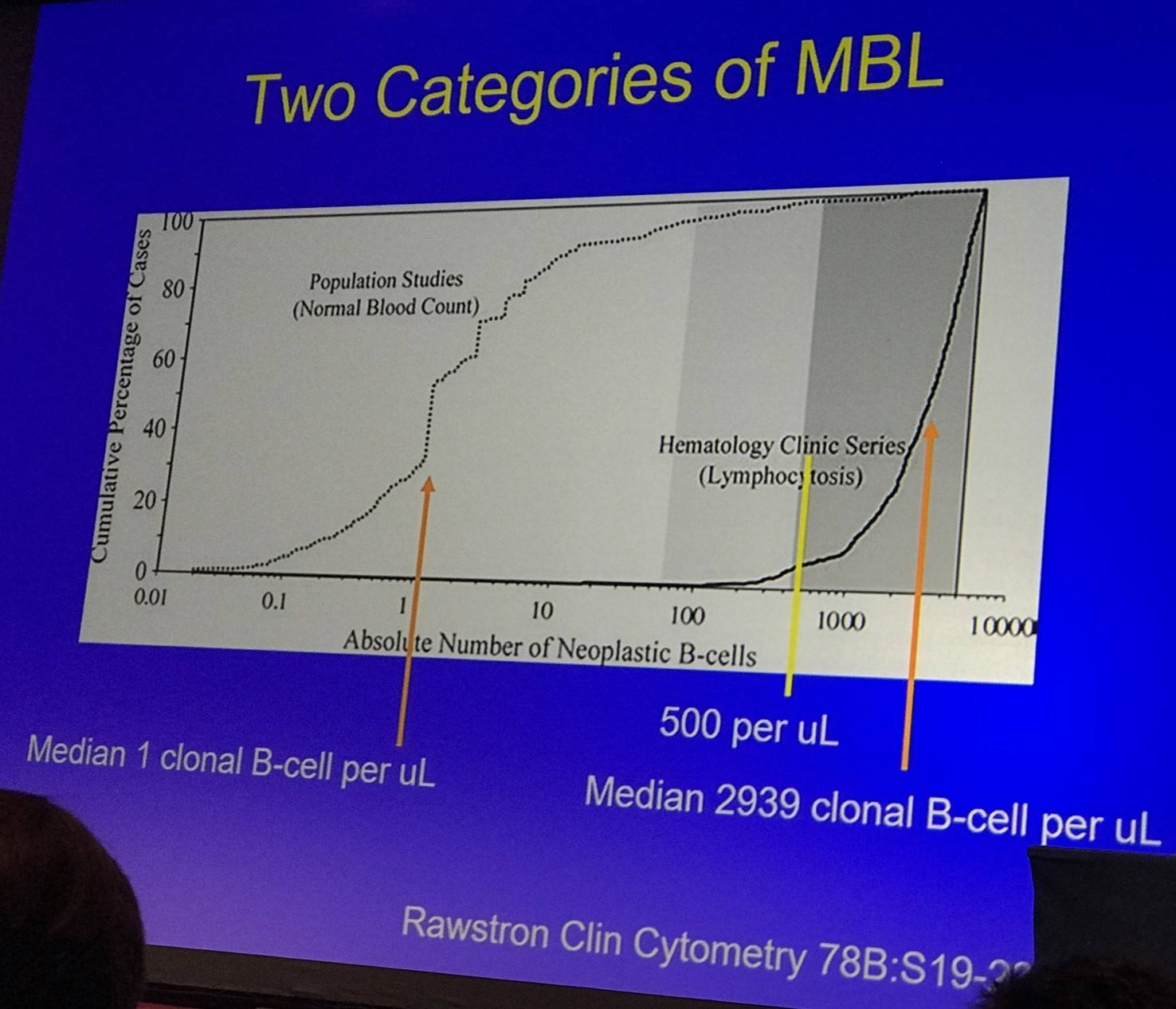
The talk then focused on findings published by Landgren et al., who conducted a PLCO screen trial in 77,469 healthy adults. Of these, 129 developed CLL. Pre-diagnosis samples were available for 45 cases and using 6 color flow cytometry and IGHV analysis by RT-PCR found that 44/45 had pre-existent MBL up to 6.4 years prior to CLL, and was present in both mutated and unmutated IGHV cases.
Tait Shanafelt moved on to discuss the natural history of LC- (Fazi et al. 2011) and HC-MBL (Rawstron et al. 2008):
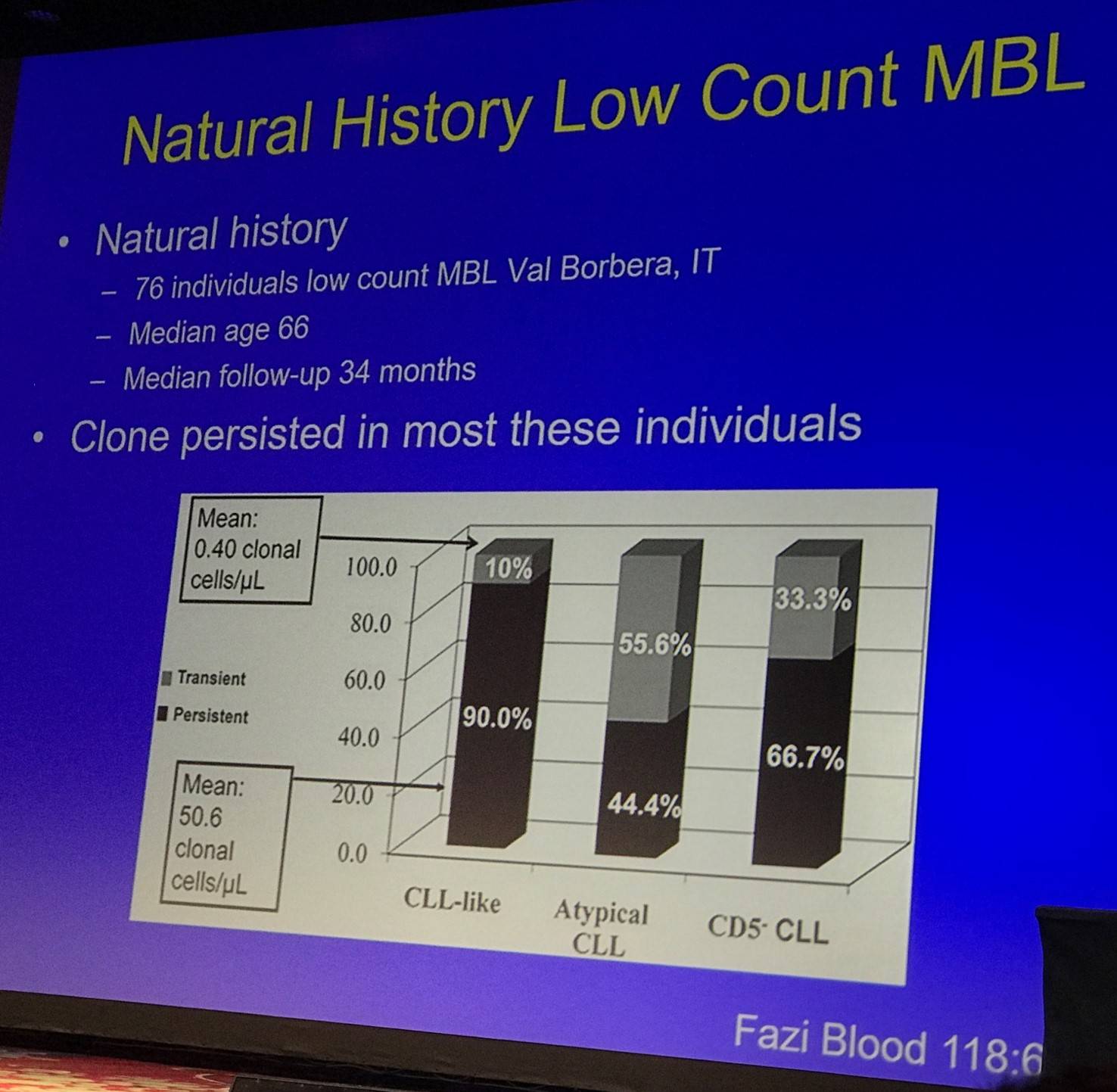
Fazi et al. also found no increase in size of the B-cell clone, and no patients developed clinical leukemia (including two patients with del(17p13) in more than 80% of clonal cells at baseline).
For HC-MBL, Rawstron et al. followed 185 patients:
- 28% developed progressive lymphocytosis
- 15% subsequently met criteria for CLL (after median 6.7 years)
- 7% required chemotherapy
- 2% died of CLL
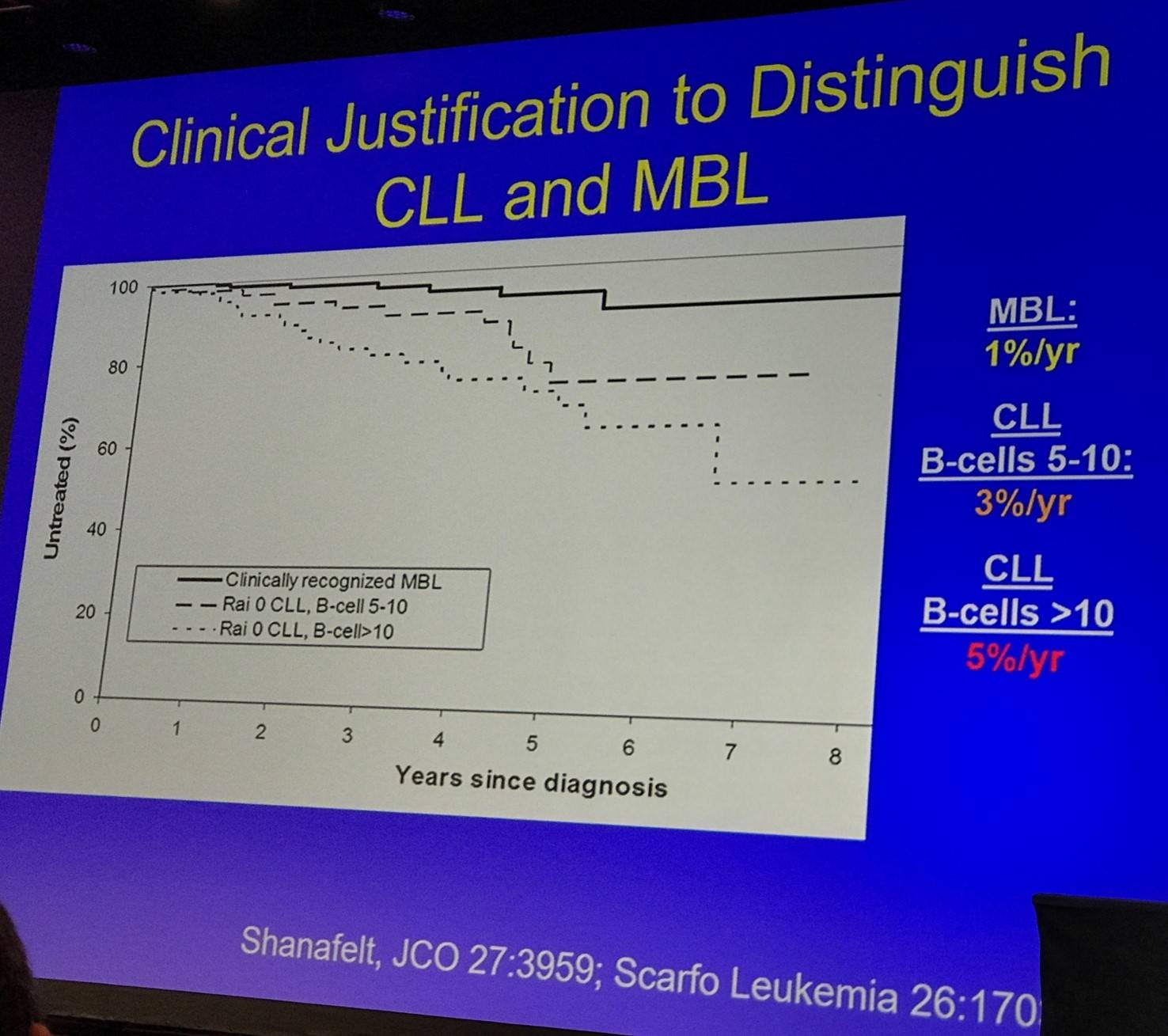
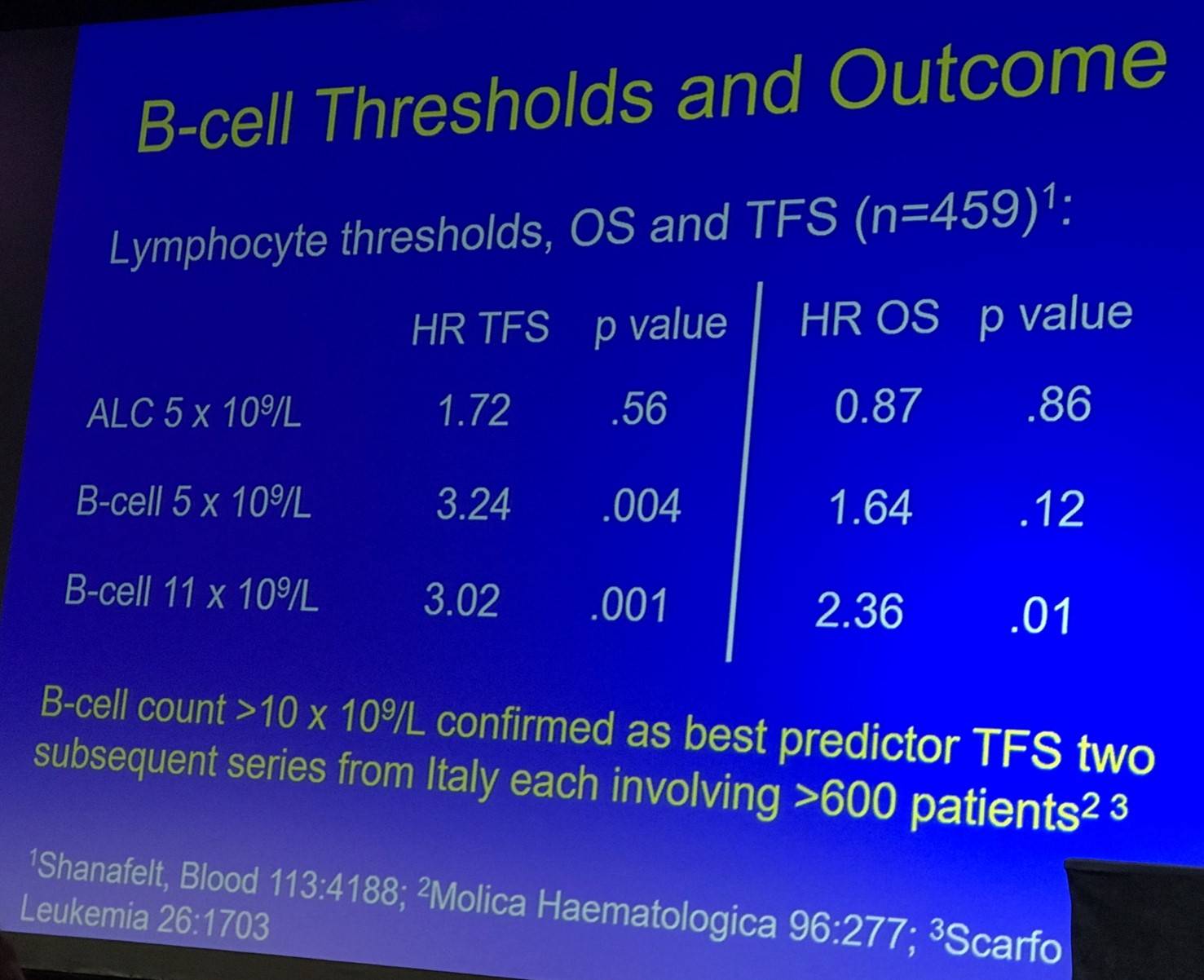
The prognosis of HC-MBL was then outlined:
- B-cell count is the single most important parameter (independent other prognostic markers, belongs in classification schema)
- Prognostic profile similar to Rai 0 CLL
- Studies typically suggest that markers which predict outcome for CLL also predict Treatment Free Survival (TFS) in HC-MBL: ZAP70, CD38, IGHV, FISH, CD49d
- The role of prognostic parameters in routine practice is debatable (due to low rates of progression of HC-MBL)
Biologic Insights
Shanafelt then discussed the genetic susceptibility of MBL. Genome-Wide Association Studies (GWAS) have found around 40 susceptibility loci to CLL, which explain approximately a quarter of familial risk of CLL. In a pooled analysis of 342 HC-MBL and 77 LC-MBL cases identified at least 9 of these loci also associated with risk of MBL (individuals with 6 or more risk alleles had a 3-fold increased risk of MBL).
The talk then discussed the IGHV repertoire and genetics (FISH and sequencing) of LC- and HC-MBL:
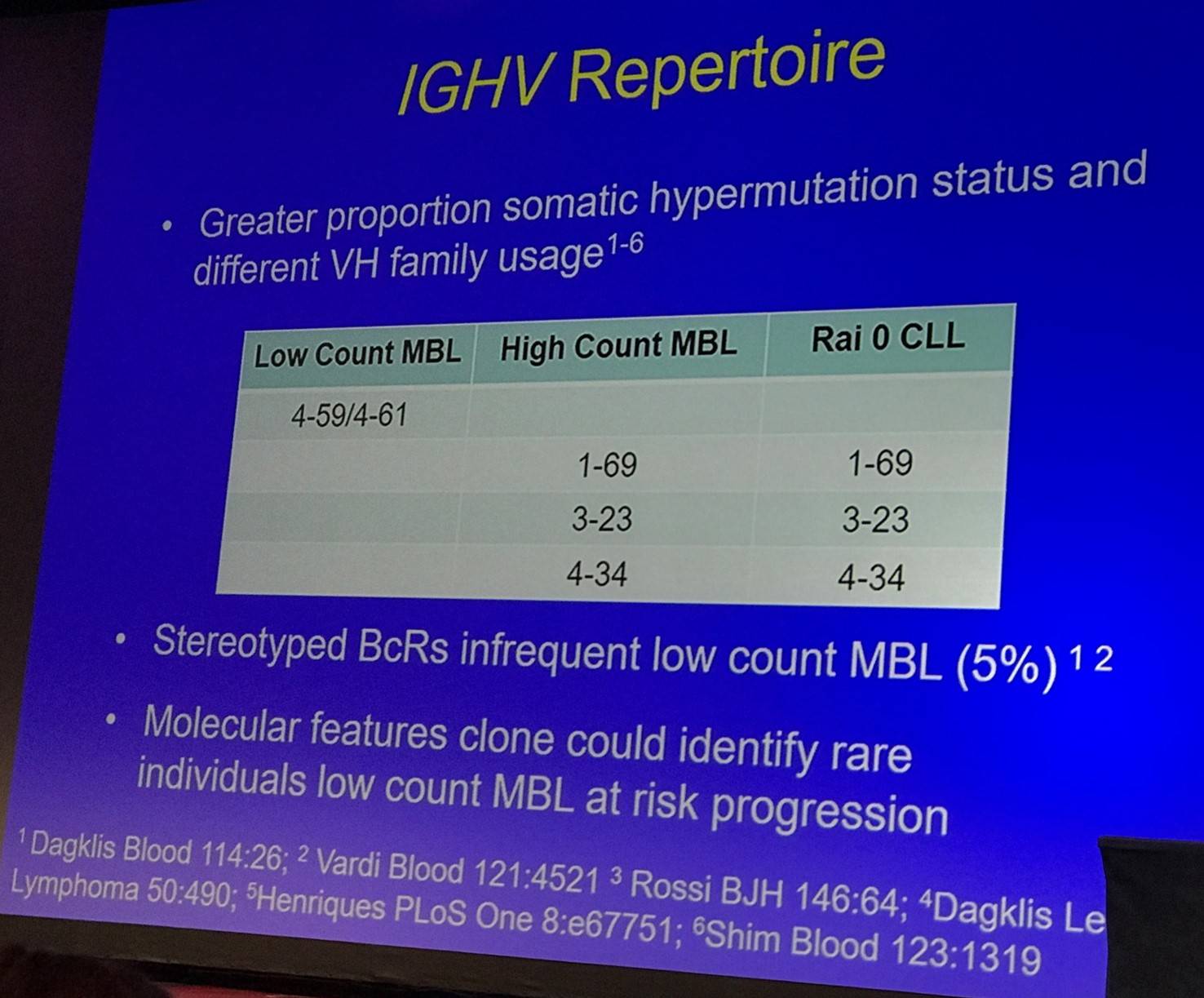
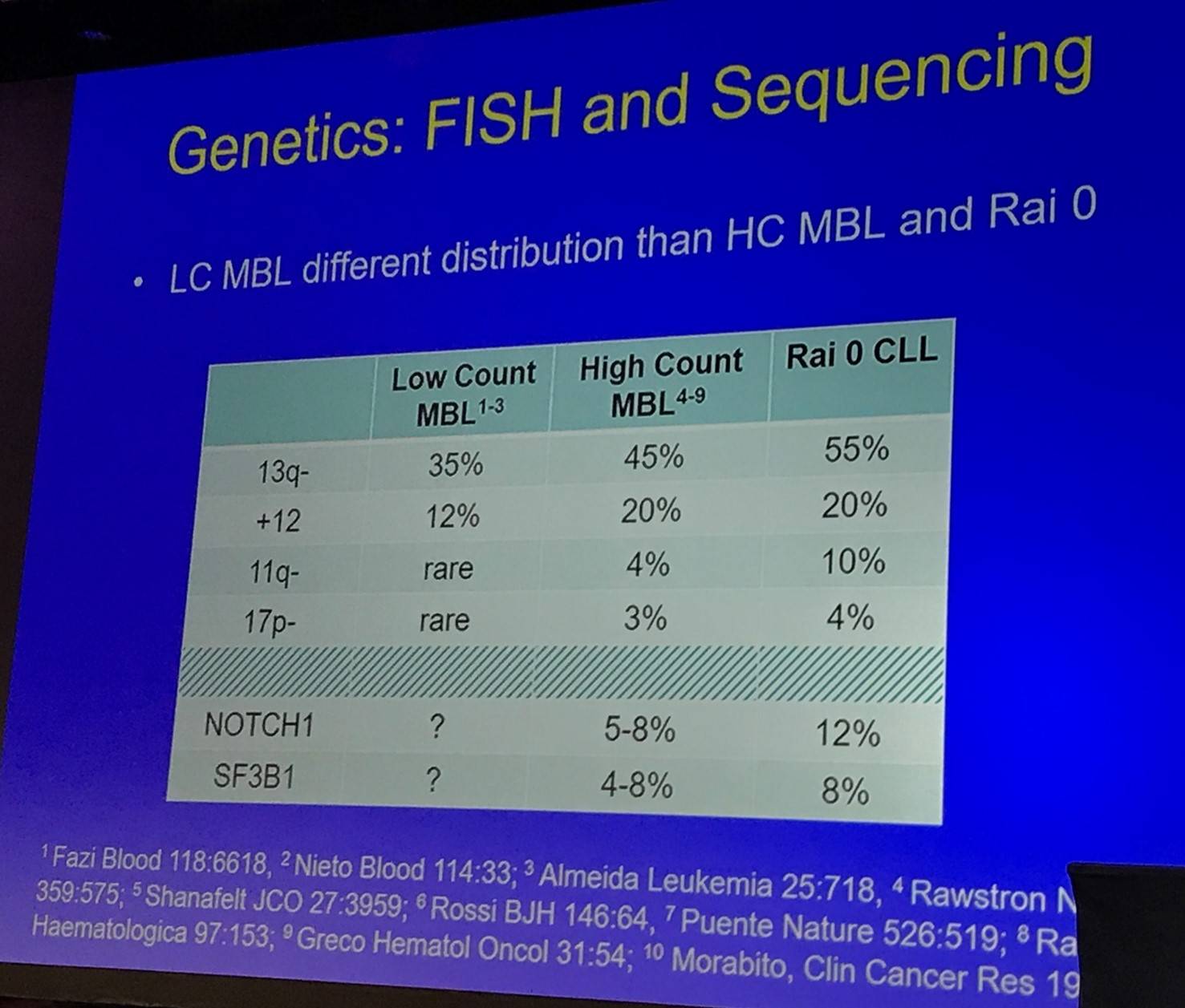
Tait Shanafelt then outlined the longitudinal aspects of sequencing:
- HC-MBL cases are indistinguishable from CLL after accounting for IGHV category at the genomic, transcriptomic, and epigenomic levels
- Whole exome/targeted sequencing of 48 MBL cases at two time points (median 55 months apart) found that most mutations present at the second time point are detected at the first time point; identified genetic sub-clones at baseline
- MBL cases with mutations in driver genes at baseline have a shorter time to treatment (Barrio et al. 2016)
Immune Dysfunction
This portion of the talk began by outlining immunoglobulin levels in MBL (Criado et al. 2017):
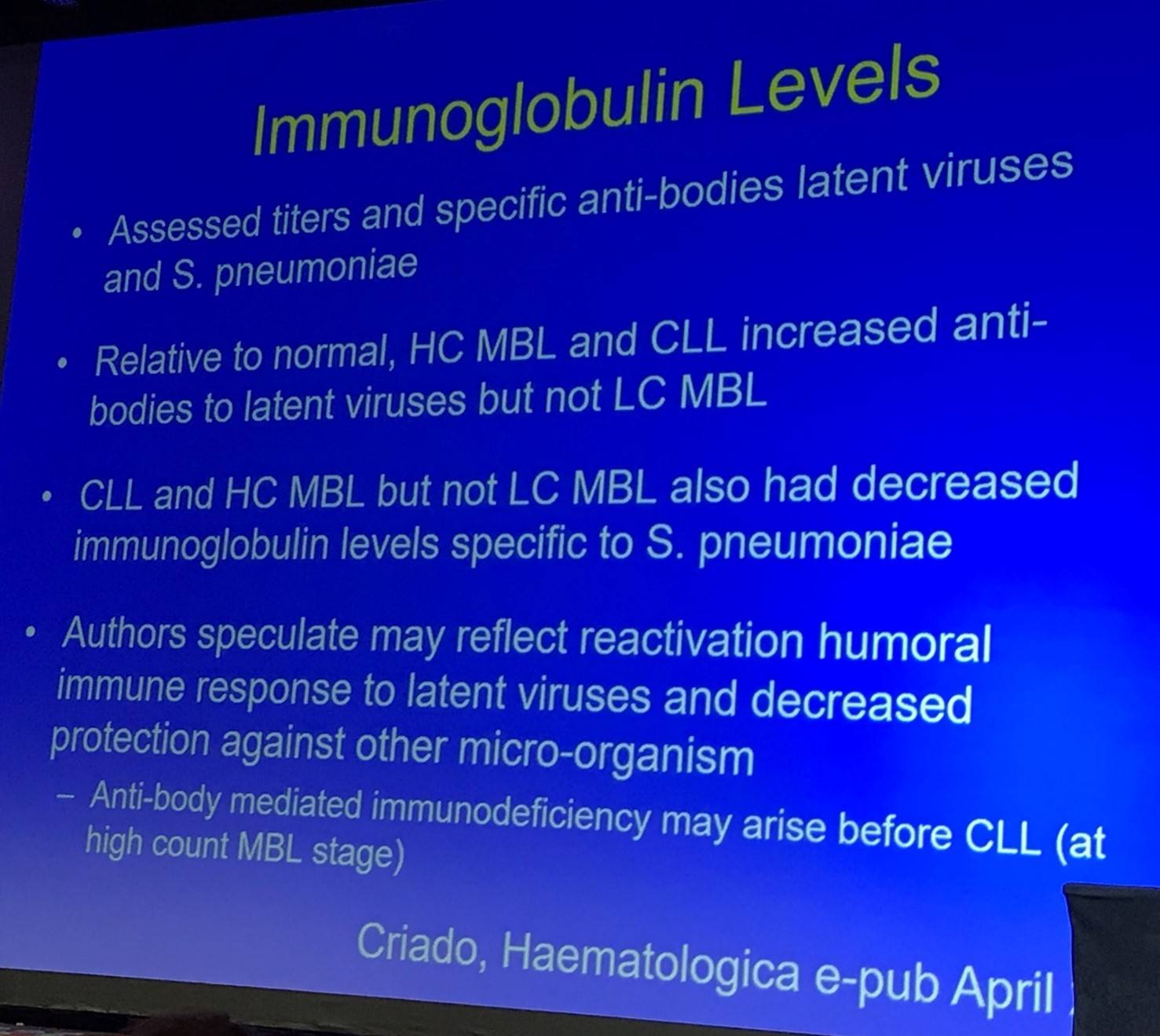
The T-cell compartment of MBL was then discussed:
- Total T-cells and T-cell subsets (CD4, CD8, memory, effector T-reg) are not expanded in HC-MBL
- Clonal T-cell populations (mostly CD4/CD8 double positive subset) are observed in 75% of LC-MBL cases
- Longitudinal analysis of T-cell subsets in HC-MBL individuals found an increase in exhausted T-cells (CD8+CD160+) compared to age-matched individuals and those who progressed had a higher proportion of exhausted T-cells at baseline
- Immunological T-cell synapse function was worse in those with MBL vs. controls
- T-cell synapse function became progressively worse as B-cell clone expanded (Parikh et al. EHA abstract P580)
Shanafelt then discussed infections in patients with HC-MBL:
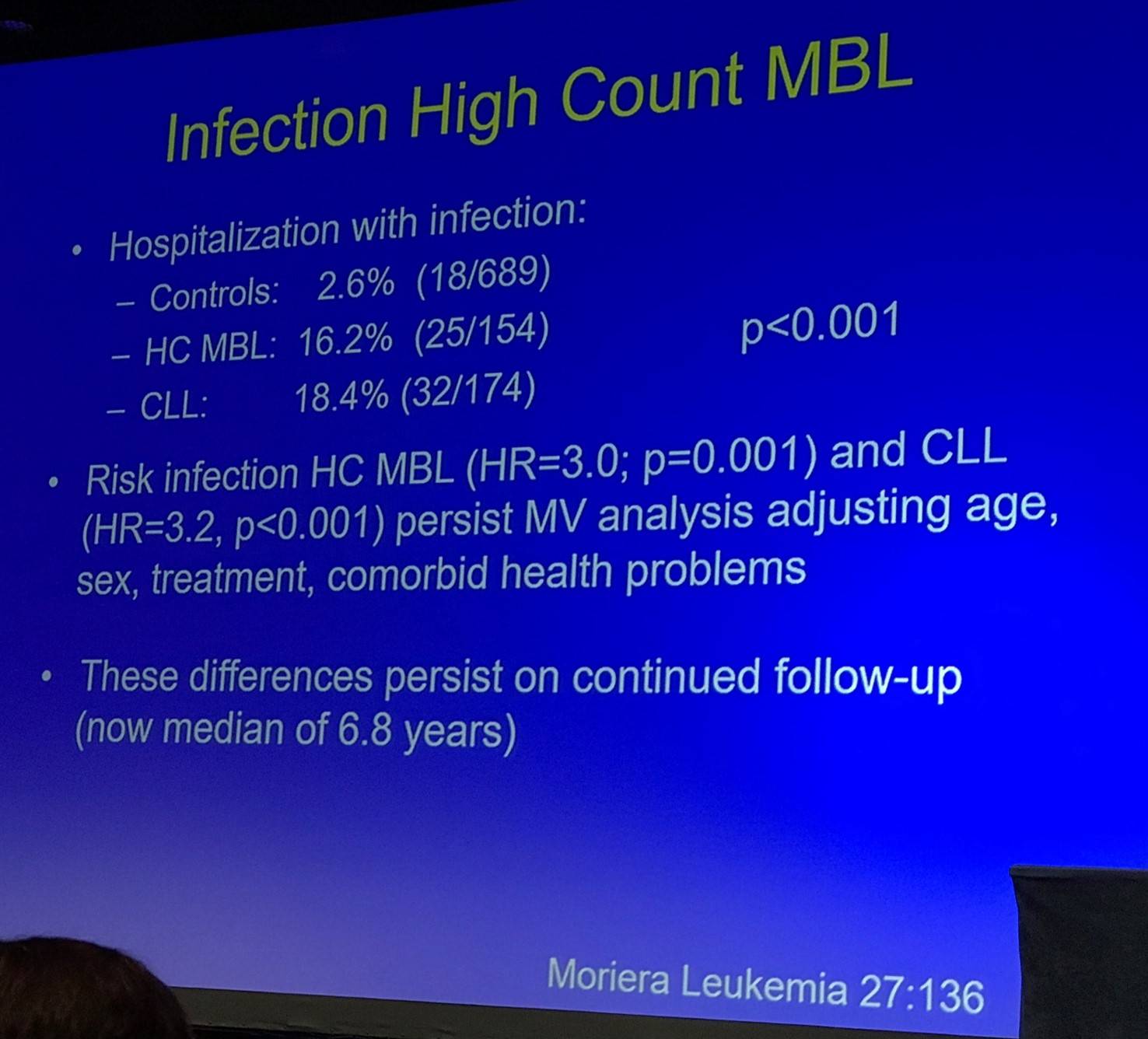
HC-MBL is also associated with an increased risk of non-hematologic cancer (Solomon et al. 2015):
- Increased risk persisted after excluding concurrently diagnosed cases (P = 0.006)
- Risk of cancer: MBL HR = 2.47 (P = 0.02); CLL HR = 2.1 (P = 0.04)
- Cases with HC-MBL had increased risk of non-hematologic cancer and serious infection; these risks led to a greater risk of progression to CLL which required treatment
Infection in LC-MBL has been evaluated at the Mayo Clinic. Of patients aged 40 years or older and identified to have CLL-Like MBL (79/672; 12%) by 8 color flow cytometry, 55 required hospitalization for infection. The HR for hospitalization with infection among cases of LC-MBL = 2.04 (95% CI, 1.05–2.94).
Conclusion
Tait Shanafelt finished his presentation with a concise summary slide:
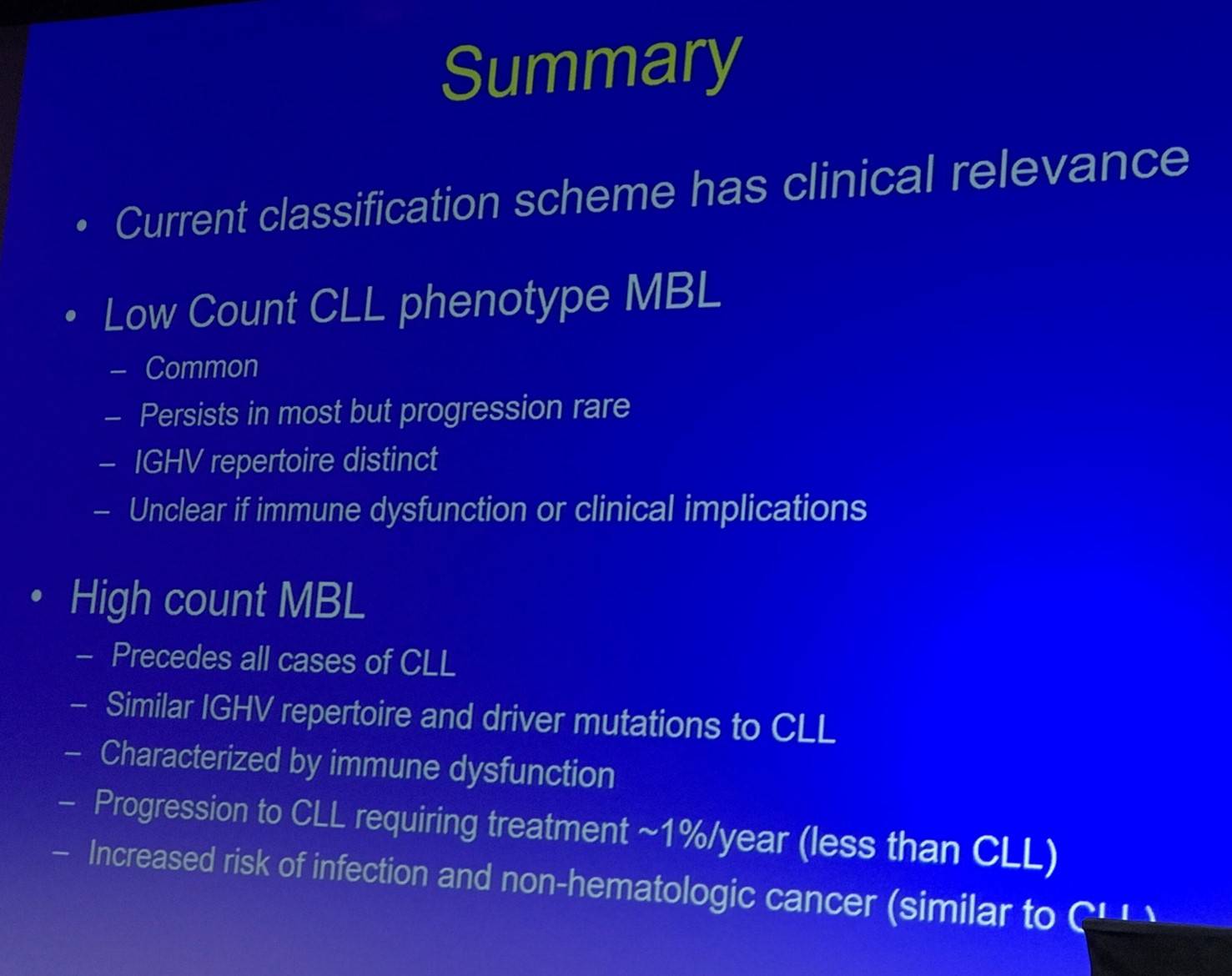
- Shanafelt T. Monoclonal B-Lymphocytosis: Recent Clinical and Biologic Insights. XVII International Workshop on Chronic Lymphocytic Leukemia; 2017 May 12–15; New York, USA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








