All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
EHA-SWG 2017 | Rare Lymphomas: biology of Central Nervous System (CNS) Lymphoma
Bookmark this article
On March 10–12 2017, the EHA-SWG meeting on Rare Lymphomas took place in Barcelona, Spain, and was jointly chaired by Prof. Martin Dreyling, from Klinikum der Universität München, Germany, and Prof. Maire José Kersten, from the Academic Medical Center, Amsterdam, The Netherlands.
On March 10th 2017, D. de Jong (VU University Medical Center, Amsterdam, The Netherlands) gave a talk during the CNS Lymphoma scientific session, which focused on the biology of CNS Lymphomas.
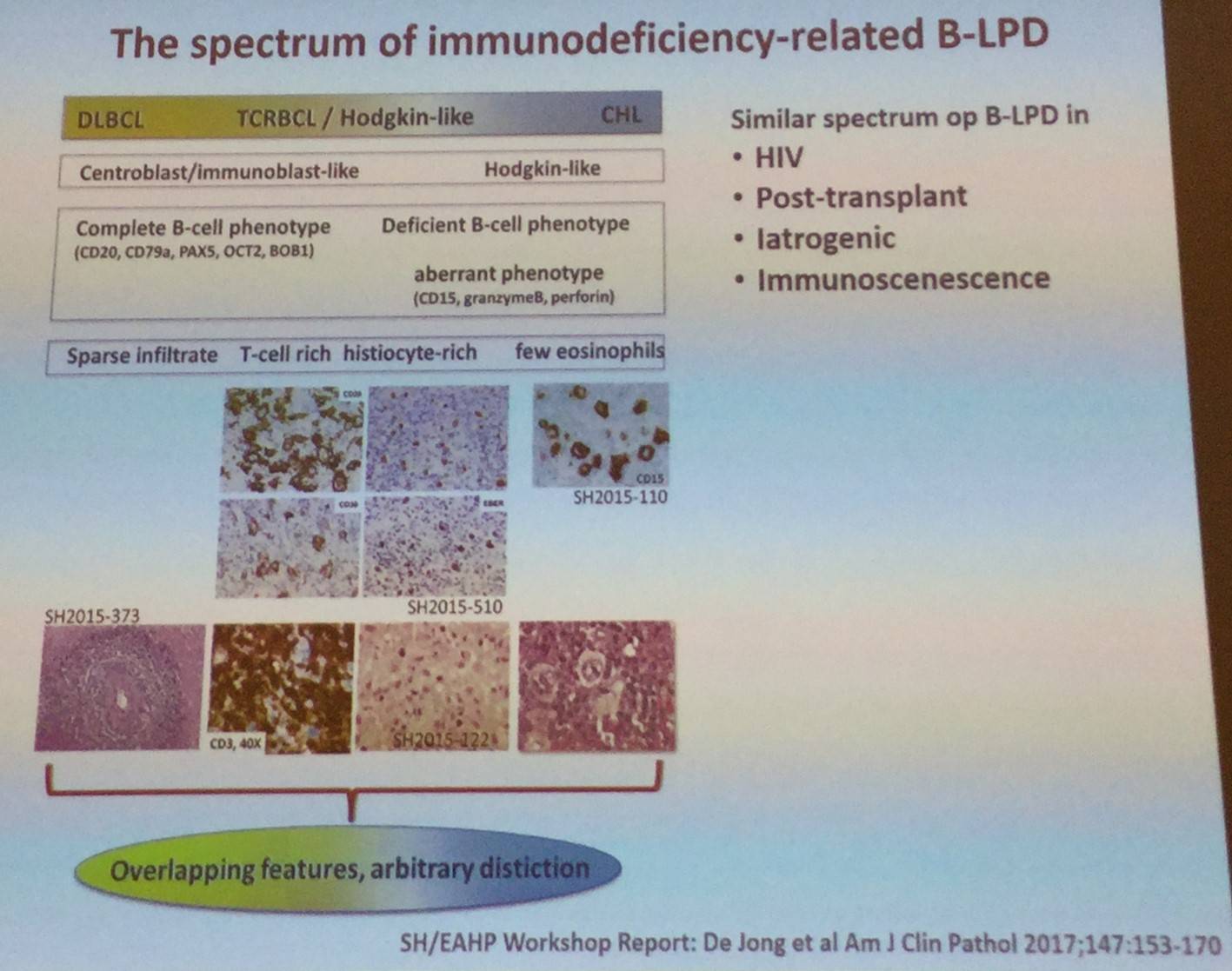
The talk began by explaining that immunodeficiency-related Lymphoproliferative Disorders (LPDs) make up a heterogeneous group; ranging from self-limiting hyperplasias to aggressive lymphomas. The WHO classification of immunodeficiency-related LPDs (2008 and 2016) is structured around 4 major categories, based on the immunodeficiency setting/background in which they arise: Post-Transplant Lymphoproliferative Disorders (PTLDs), lymphomas associated with Human Immunodeficiency Virus (HIV) infection, LPDs associated with primary immune disorders, and other iatrogenic immunodeficiency-associated LPDs.
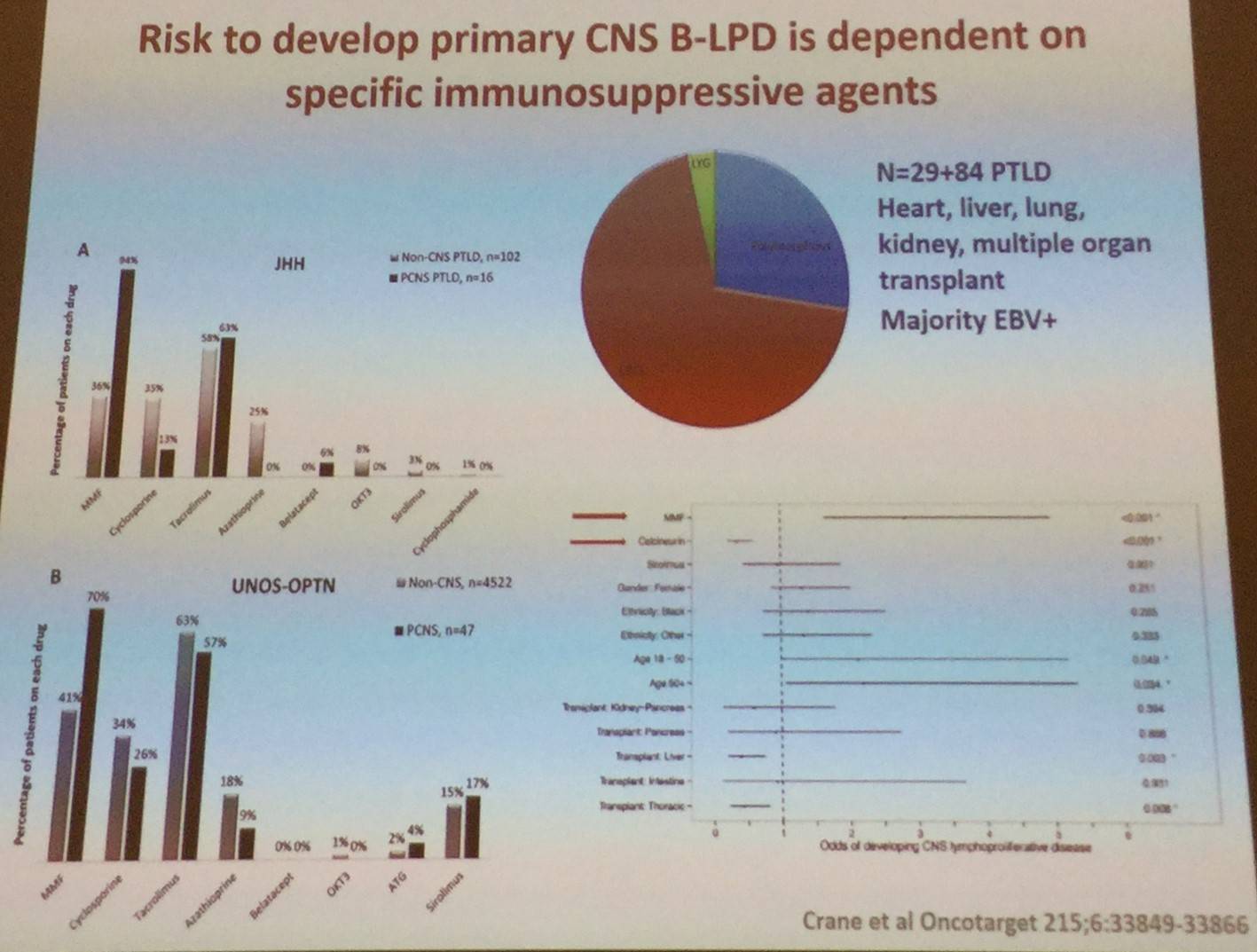
It has been previously reported that immunosuppression for solid organ transplantation increases risk of LPD. Crane et al. (Oncotarget 2015) identified all PTLDs diagnosed over a 28-year time frame at their institution (total = 174 total, PCNS = 29) and all similar cases captured in the United Network for Organ Sharing-Organ Procurement and Transplant Network (UNOS-OPTN) data file.
- Between 1986 and 1997, no PCNS cases were diagnosed
- PCNS cases made up 37% of PTLDs diagnosed between 2011–2014
- PCNS disease was more frequently associated with renal vs other organ transplant, EBV, large B-cell morphology, and Mycophenolate Mofetil (MMF) when compared to PTLD that did not have CNS involvement
- Calcineurin inhibitors were protective against PCNS disease when given alone or in combination with MMF
- Multivariate analysis of UNOS-OPTN dataset found that MMF and lack of calcineurin inhibitor usage were independently associated with risk for development of PCNS PTLD
- These results carry significant implications for the transplant therapy, particularly in the introduction of new therapeutic strategies which lack calcineurin inhibitor usage
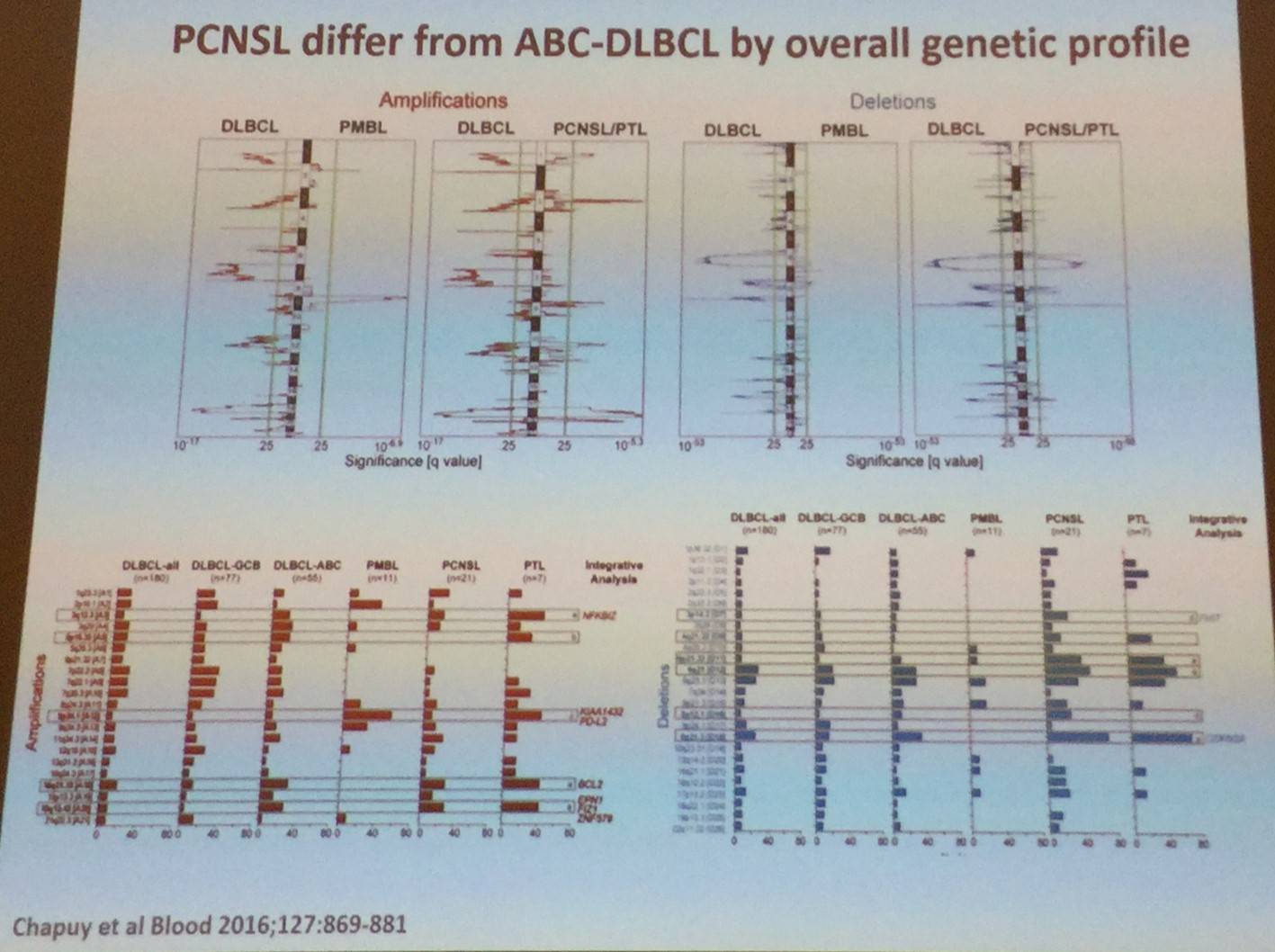
 D. De Jong then went on to state that PCNSL bears similarities to ABC-DLBCL, such as:
D. De Jong then went on to state that PCNSL bears similarities to ABC-DLBCL, such as:
- Recurrent mutations in MYD88 and CD79B
- Both frequently exhibit genomic instability and near-uniform, often biallelic, CDKN2A loss
- Rare TP53 mutations
- Both display near-uniform oncogenic Toll-Like Receptor (TLR) signaling due to MYD88 mutation and/or NFKBIZ amplification
- Frequent concurrent B-Cell Receptor (BCR) activation and deregulation of BCL6
- PCNSLs and PTLs often have 9p24.1/PD-L1/PD-L2 copy number alterations and additional translocations of these loci
However, PCNSL does differ from ABC-DLBCL by its overall genetic profile:
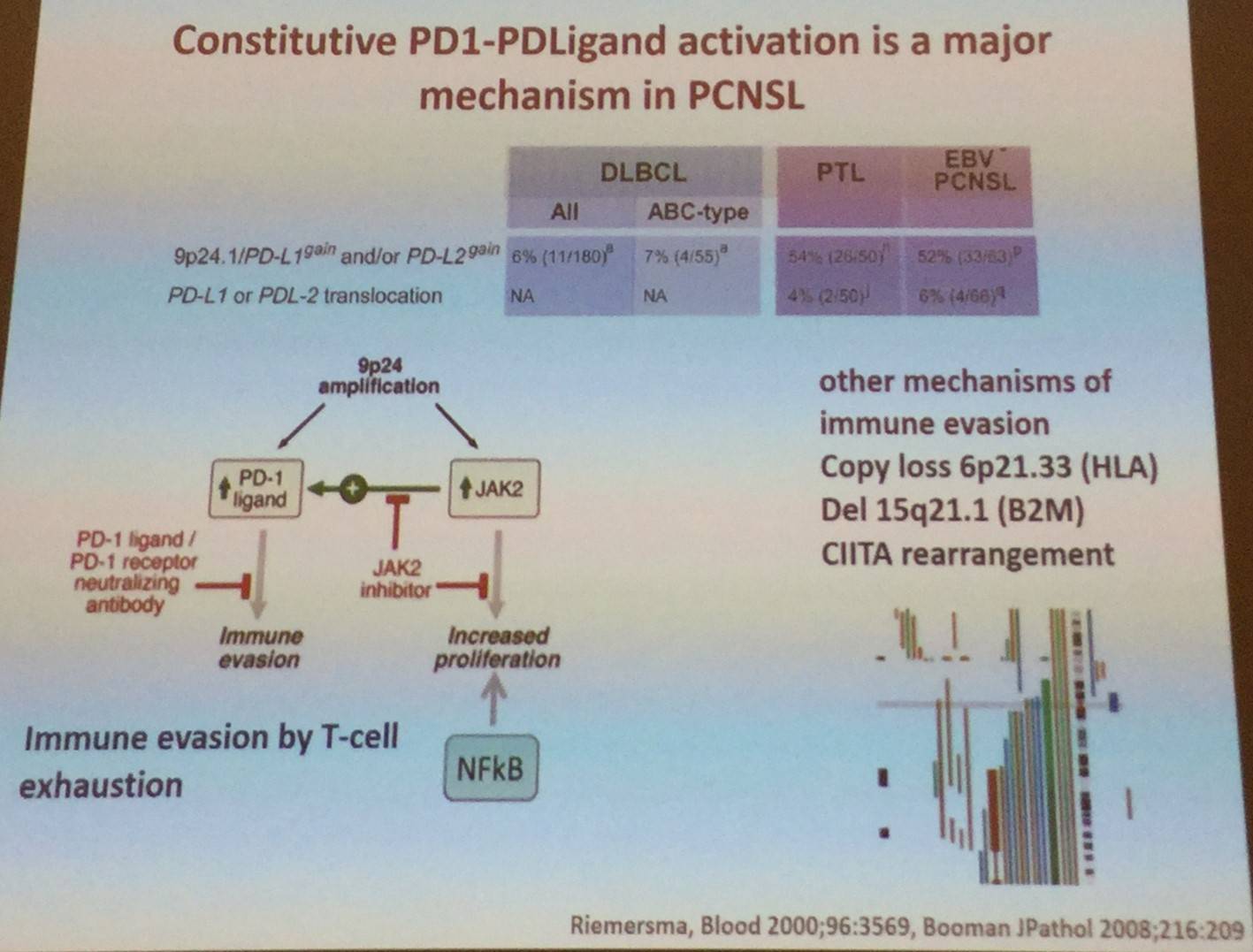
It was also explained that constitutive TLR and BCR signaling are major mechanisms of PCNSL: NFKBIZ gain on chromosome 3 occurs in 40–60% of cases and functional studies highlight NFKBIZ as a functional signaling mechanism. D. de Jong also went on to say that translocations in MYC and BCL2 are extremely rare in PCNSL. Translocations do occur with BCL6 in 17–47% of cases, pairing with Ig- and non-Ig partners. It was also stated that constitutive activation of PD-1 and PD-ligand signaling is a key mechanism of immune evasion in PCNSL.
Moreover, D. de Jong stated that loss of β2M and HLA-I are not specific to PCNSL. HLA expression is lost in >50% of PCNSL cases. Moreover, PCNSL-tumor-Ig have a restricted IgV-spectrum and recognize self-antigens expressed in glial cells. VH-mutation pattern reveals extensive signs of somatic hypermutation with signs of antigen selection.
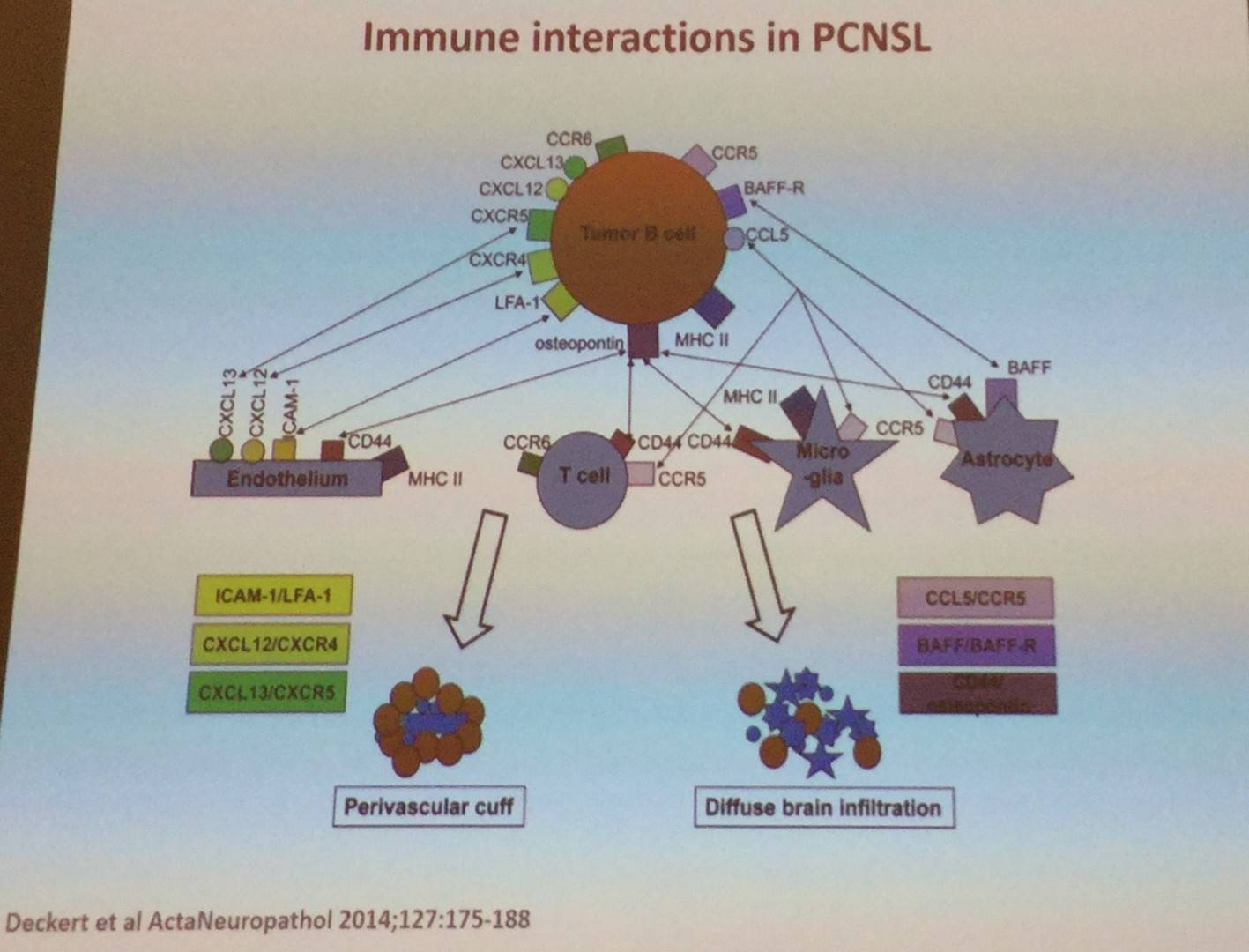
- De Jong D. Biology of CNS Lymphoma. 2017 Mar 10. EHA-SWG Rare Lymphomas. Barcelona, Spain.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








