All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
4-year results from the phase III MURANO study
The phase III MURANO study aimed to investigate the efficacy and safety of venetoclax plus rituximab (VenR) compared with bendamustine plus rituximab (BR) in patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL).1
Results from this trial, after a median follow-up of 36 months, were reported here and showed superior progression-free survival (PFS) and overall survival (OS) with VenR compared with BR. In a recent publication in the Journal of Clinical Oncology, Arnon Kater and colleagues reported outcomes after a 4-year follow-up, including responses to subsequent therapies, the predictive value of measurable residual disease (MRD) status, and genetic characteristics.1
Study design
Study design and treatment regimens have been reported previously. Briefly, 389 patients with R/R CLL were randomized (1:1) to receive either:
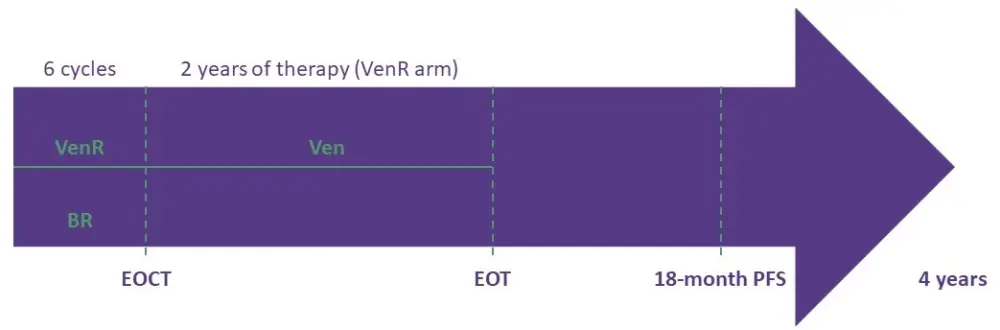
- VenR (n = 194), six 28-day cycles, followed by single-agent venetoclax (400 mg) once daily for a total of 2 years
- BR (n = 195), six 28-day cycles
Patients with progressive disease (PD) after completing treatment were eligible to receive fixed-duration VenR (approximately 2 years). The study timeline is reported in Figure 1.
Figure 1. Study timeline1
Endpoints
Primary efficacy endpoint: investigator-assessed PFS.
Secondary endpoints:
- MRD status, assessed in peripheral blood at Cycle 4, 2 to 3 months after end of combination therapy (EOCT), and then every 3 to 6 months (undetectable MRD [uMRD] was defined as < 1 CLL cell/10,000 leukocytes [< 10−4]; low MRD positivity was defined as 10−4–10−2, and high MRD positivity was defined as ≥ 10−2)
- OS
- Complete response
- Partial response
- Safety
Results
Patient baseline characteristics, as previously reported, were similar between the two arms. The median follow-up was 48 months.
PFS and OS
At the 4-year follow-up, the benefit of VenR versus BR was retained, with respect to both PFS (hazard ratio [HR], 0.19; 95% confidence interval [CI], 0.14–0.25; p < 0.0001) and OS (HR, 0.41; 95% CI, 0.26–0.65; p < 0.0001)
4-year PFS and OS rates with VenR vs BR were:
- PFS, 57.3% (95% CI, 49.4–65.3) vs 4.6% (95% CI, 0.1–9.2)
- OS, 85.3% vs 66.8%
PFS estimates for patients who completed venetoclax treatment without developing PD (n = 130) were 75.5% (95% CI, 67.4–83.7) at 18 months and 68.0% (95% CI, 57.6–78.4) at 24 months.
Best responses in patients who received novel agents after developing PD are reported in Table 1. In the VenR arm vs the BR arm, the median time on next therapy was 8.7 months (range, 0.7–42.9; 17 of 28 patients still on treatment) vs 16.5 months (range, 0.03–47.4; 47 of 81 patients still on treatment)
Table 1. Best response by treatment subgroup1
|
BR, bendamustine plus rituximab; BTKi, Bruton tyrosine kinase inhibitor; CR, complete response; CRi, CR with incomplete marrow recovery; NA, not available; nPR/PR, partial response with or without nodes; PD, progressive disease; PI3Ki, phosphoinositide 3-kinase inhibitor; SD, stable disease; VenR, venetoclax plus rituximab. |
|||||||||||
|
Treatment |
BR (n = 81) |
VenR (n = 28) |
|||||||||
|
PD |
SD |
nPR/PR |
CR/CRi |
NA |
PD |
SD |
nPR/PR |
CR/CRi |
NA |
Nonresponder |
|
|
BTKi |
3 |
7 |
30 |
5 |
15 |
0 |
0 |
9 |
1 |
2 |
0 |
|
PI3Ki |
1 |
0 |
6 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
|
Venetoclax |
0 |
1 |
1 |
2 |
6 |
2 |
2 |
6 |
0 |
3 |
1 |
|
Other |
0 |
0 |
2 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
The response rate was 100% among evaluable patients (10 out of 10) treated with ibrutinib after venetoclax and 55% among evaluable patients (6 out of 11) treated with a venetoclax-based regimen after venetoclax therapy.
MRD
Superior outcomes were observed in patients who achieved uMRD at the EOCT, compared with patients who remained MRD positive. HRs for PFS in the VenR arm and the BR arm are reported in Table 2.
Table 2. HRs for PFS in both study arms based on MRD status at EOCT1
|
BR, bendamustine plus rituximab; EOCT, end of combination therapy; HR, hazard ratio; MRD, measurable residual disease; PFS, progression-free survival; uMRD, undetectable MRD; VenR, venetoclax plus rituximab. |
|||
|
Arms |
Category |
HR (95% CI) |
p value |
|
VenR |
uMRD vs low MRD positivity |
0.50 (0.28–0.89) |
0.02 |
|
uMRD vs high MRD positivity |
0.15 (0.06–0.36) |
< 0.0001 |
|
|
Low MRD positivity vs high MRD positivity |
0.25 (0.10–0.66) |
0.002 |
|
|
BR |
uMRD vs low MRD positivity |
0.64 (0.36–1.11) |
0.1 |
|
uMRD vs high MRD positivity |
0.10 (0.05–0.21) |
< 0.0001 |
|
|
Low MRD positivity vs high MRD positivity |
0.25 (0.15–0.42) |
< 0.0001 |
|
For patients in the VenR arm who completed 2 years of venetoclax, 18-month PFS rates from end of treatment (EOT), according to MRD status at EOT were:
- 90.3% (95% CI, 83.5–97.2) in patients with uMRD
- 64.4% (95% CI, 42.1–86.6) in patients with low MRD positivity
- 8.3% (95% CI, 0.0–24.0) in patients with high MRD positivity
Molecular biomarkers
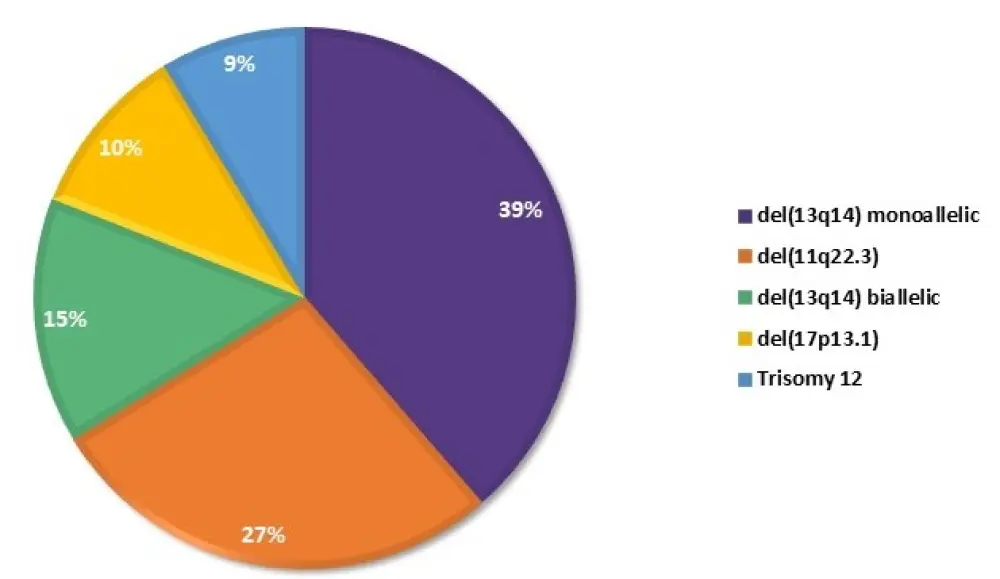
The rates of major genomic aberrations, detected at baseline by high-density array comparative genomic hybridization (aCGH) in the biomarker-evaluable population of the VenR arm (n = 142), are reported in Figure 2.
Figure 2. Rates of the major genomic aberrations, detected at baseline in the biomarker-evaluable population of the VenR arm1
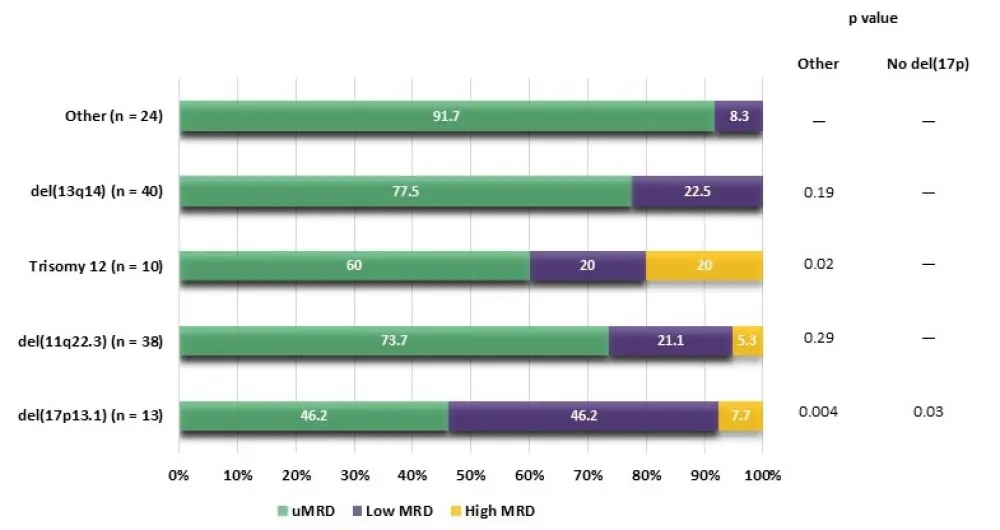
At EOCT, MRD positivity (low or high) was more frequent in patients with del(17p) vs those without del(17p) (p = 0.031) or without any of the four major alterations (p = 0.004)1, using Döhner hierarchical classification2. An association was also found between MRD-positive status at EOCT and trisomy 12 (p = 0.024; Figure 3).1
Figure 3. MRD status at EOCT according to major cytogenetic alterations, using Döhner hierarchical classification.1
No association with MRD status was observed at EOT for any of the major alterations. In patients with cytogenetic alterations, PFS rates were higher with VenR than BR in all molecular subsets.
Of 288 patients with genomic complexity (GC) status data:
- 194 (67.3%) had non-complex (non-GC) status (0─2 aberrations)
- 63 (21.9%) had low GC (3─4 aberrations)
- 31 (10.8%) had high GC (≥ 5 aberrations)
A correlation was observed between high- and low-GC status and increased frequency of high MRD positivity at EOT (p = 0.042). In addition, although VenR showed superiority compared with BR in each GC category, patients in the VenR arm with non-GC had better PFS than those with high-GC (HR, 2.9; 95% CI, 1.1─3.6; p = 0.0057) or low-GC status (HR, 2.0; 95% CI, 1.4─6.3; p = 0.025). A similar pattern was observed in the BR arm; patients with non-GC status had better PFS than those with high-GC (HR, 1.9; 95% CI, 1.1─3.2; p = 0.02) or low-GC status (HR, 1.7; 95% CI, 1.0─2.7; p = 0.039).
The association between mutations in commonly mutated genes (in ≥ 5% of patients) in the VenR arm and MRD status at EOT was also assessed: lower uMRD rates were reported in patients with TP53, NOTCH1, XPO1, and BRAF mutated genes.
Safety
Since the previous analysis, no new serious adverse events related to the study drug were reported.
Conclusion
The efficacy benefits of fixed-duration VenR vs BR in patients with R/R CLL were sustained over 48 months. Patients with uMRD status at EOT had superior PFS than patients with low or high MRD positivity. In addition, patients with high- and low-GC status had an increased frequency of high MRD positivity at completion of venetoclax therapy and inferior PFS, compared with the non-GC group. High response rates to salvage therapy were observed in patients who developed PD after VenR treatment, especially with ibrutinib. The small sample size for specific subsets in these analyses calls for further studies in order to confirm these findings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

