All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
6-year follow-up results of the CLL14 study of venetoclax-obinutuzumab in previously untreated CLL
The CLL14 trial (NCT02242942), comparing the safety and efficacy of fixed-duration venetoclax plus obinutuzumab (Ven-Obi) against chlorambucil plus obinutuzumab (Clb-Obi) treatment in patients with treatment-naïve chronic lymphocytic leukemia (CLL) and comorbidities, is ongoing. This is the first trial to compare a novel, chemotherapy-free, fixed-duration regimen with chemoimmunotherapy for patients with treatment-naïve CLL, including those with del17p or TP53 mutations.1
The Lymphoma Hub previously reported on the long-term safety outcomes of Ven-Obi vs Clb-Obi in patients with CLL at a median follow-up of 39.6 months. Below, we summarize the 6-year efficacy and safety results of the CLL14 trial in previously untreated CLL, presented by Al-Sawaf at the European Hematology Association (EHA) 2023 Congress.2
Study design1,2
CLL14 is an ongoing multicentre, randomized, open-label phase III trial that included patients aged ≥18 years with previously untreated CLL and comorbidities (cumulative illness rate of ≥6 or creatinine clearance of 30–69 mL/min). Patients were randomized 1:1 to receive either 12 cycles of venetoclax with six cycles of obinutuzumab (Ven-Obi arm), or 12 cycles of chlorambucil with six cycles of obinutuzumab (Clb-Obi arm).
The primary endpoint was investigator-assessed progression-free survival (PFS). Key secondary endpoints included response, minimal residual disease (MRD), and overall survival (OS).
Results
In total, 432 patients were treated, 216 with fixed-duration Ven-Obi and 216 with Clb-Obi. Baseline characteristics were summarized in Table 1.
Table 1. Baseline characteristics*
|
Clb-Obi, chlorambucil plus obinutuzumab; CIRS, cumulative illness rating score; del, deletion; IGHV, immunoglobulin heavy chain variable region; TLS, tumor lysis syndrome; Ven-Obi, venetoclax plus obinutuzumab. |
||
|
Characteristic, % (unless otherwise stated) |
Ven-Obi |
Clb-Obi |
|---|---|---|
|
Median age, years |
72 |
71 |
|
Binet stage |
|
|
|
A |
21 |
20 |
|
B |
35 |
37 |
|
C |
44 |
43 |
|
Median total CIRS score (range) |
9 (0−23) |
8 (1−28) |
|
TLS risk category |
|
|
|
Low |
13 |
12 |
|
Intermediate |
64 |
68 |
|
High |
22 |
20 |
|
IGHV mutational status |
|
|
|
Unmutated |
61 |
59 |
|
Mutated |
38 |
40 |
|
Not evaluable |
1 |
1 |
|
Del (17p) and/or TP53 mutation |
12 |
12 |
|
Cytogenetic subgroups |
|
|
|
Deletion in 17p |
8 |
7 |
|
Deletion in 11q |
17 |
18 |
|
Trisomy in 12 |
17 |
19 |
|
No abnormalities |
24 |
20 |
|
Deletion in 13q alone |
34 |
36 |
Efficacy2
The median PFS overall and across high-risk subgroups are reported in Figure 1. At a median follow-up of 76.4 months, the 6-year PFS rate was significantly higher in the Ven-Obi vs Clb-Obi arm. Patients without TP53WT had significantly longer PFS than those with TP53 deletions/mutations across both arms. Multivariate analyses showed that a high lymph node size of ≥5 cm, an unmutated IGHV and TP53 deletions/mutations were independently prognostic of the worse PFS outcomes in the Ven-Obi arm.
Figure 1. Median PFS in Ven-obi vs Clb-Obi arm*
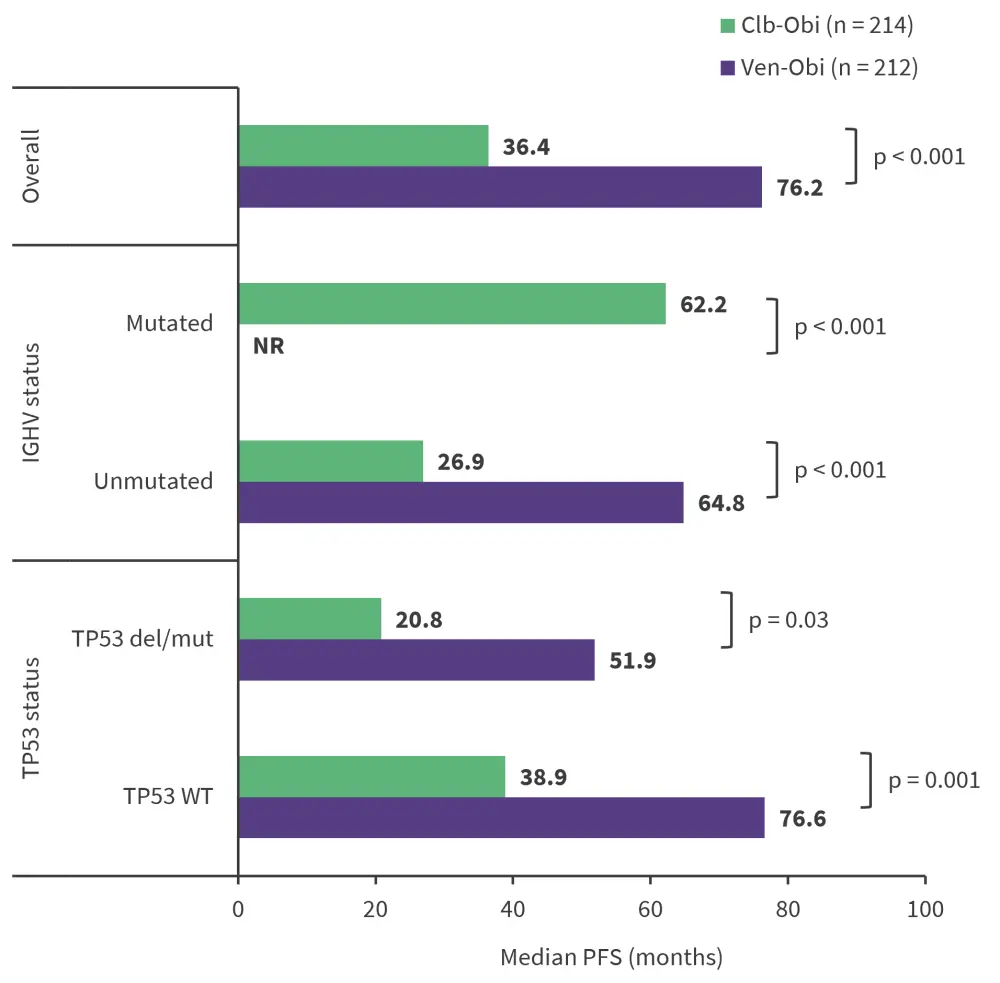
Clb-Obi, chlorambucil plus obinutuzumab; del, deletion; IGHV, immunoglobulin heavy chain variable region; mut, mutation; NR, not reached; PFS, progression-free survival; Ven-Obi, venetoclax plus Obinutuzumab; WT, wild type.
*Data from Al-Sawaf, et al.1
- The median time to the next treatment was not reached in the Ven-Obi arm but was 52.9 months in the Clb-Obi arm.
- A total of 39 patients in the Ven-Obi arm and 103 patients in the Clb-Obi required second-line treatment; most patients received targeted therapies, with 53–59% receiving Bruton’s kinase inhibitors and 15–18% receiving BCL2 inhibitors, although 23–30% of patients received chemotherapy/chemoimmunotherapy.
- The median OS was not reached in both arms and the 6-year OS was slightly higher in the Ven-Obi vs Clb-Obi arm, but this difference was not significantly different.
- The 6-year PFS, time to next treatment, and OS rates are summarized in Figure 2.
Figure 2. 6-year responses in Ven-Obi and Clb-Obi arms*
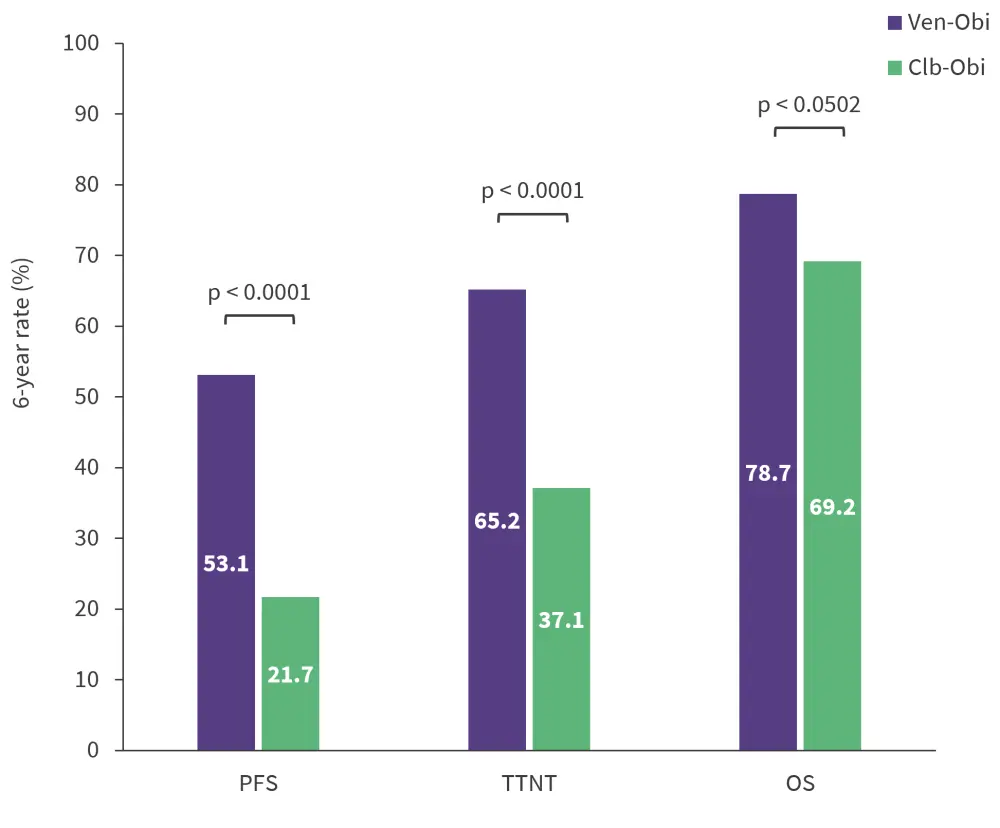
Clb-Obi, chlorambucil plus obinutuzumab; OS, overall survival; PFS, progression-free survival; TTNT, time to next treatment; Ven-Obi, venetoclax plus obinutuzumab.
*Data from Al-Sawaf, et al.1
After 5 years of treatment, 1.9% and 7.9% of patients had sustained MRD at <10−4 by next-generation sequencing in the Clb-Obi and Ven-Obi arm, respectively. In the Ven-Obi arm, the end of treatment MRD status in peripheral blood by next-generation sequencing significantly correlated with both PFS and OS, with a shorter PFS observed in patients with MRD ≥10−4 than those with <10−4.
Safety
The most common Grade ≥3 adverse events (AEs) during treatment were neutropenia, thrombocytopenia, and infusion-related reactions (Figure 3); there was a very low incidence of these Grade 3 events posttreatment. There were a higher number of overall secondary malignancy events in the Ven-Obi compared with the Clb-Obi arm, with 31 events versus 20 events, respectively, though this difference was not statistically significant.
Figure 3. Most common Grade ≥3 AEs*
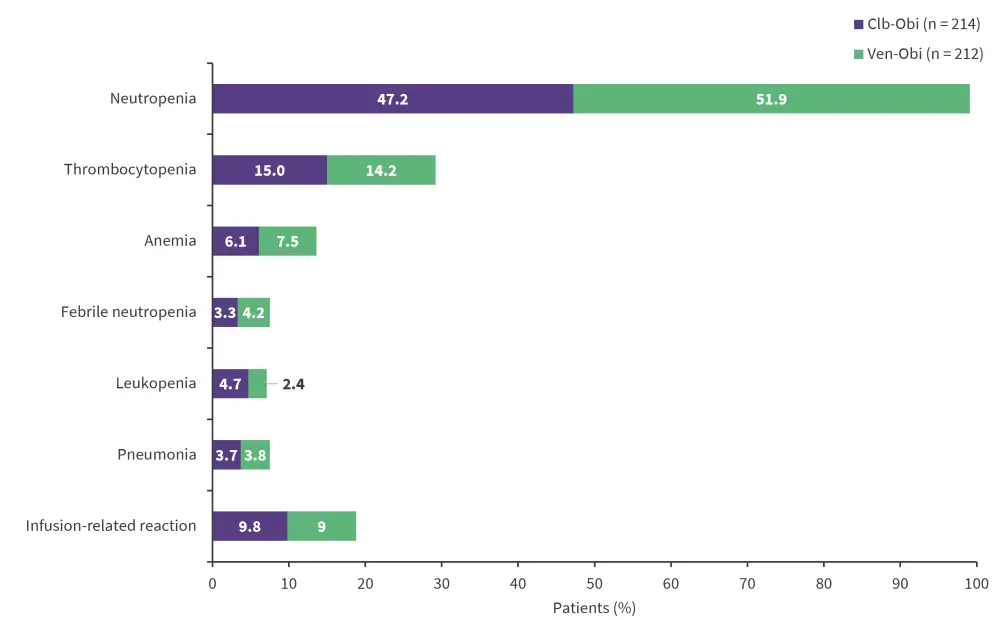
Clb-Obi, chlorambucil plus obinutuzumab; Ven-Obi, venetoclax plus obinutuzumab.
*Data from Al-Sawaf, et al.1
Conclusion
The 6-year follow-up results of CLL14 demonstrated the significant long-term PFS benefit of fixed-duration Ven-Obi across all subgroups of patients with previously untreated CLL and comorbidities. These benefits were maintained across high-risk groups, such as those harboring the TP53 deletions/mutations or IGHV unmutated, and over 60% of patients did not require a second-line treatment. This study also showed the prognostic value of end of treatment MRD status on PFS and OS, highlighting the need for MRD-guided approaches. Overall, treatment was manageable with no new safety signals reported at longer follow-up. The ongoing study continues to monitor the secondary malignancy rate with anticipated further follow-up results later this year.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


