All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
A PET-adapted approach for the treatment of early-stage bulky classic Hodgkin lymphoma
The standard care for patients with early-stage, unfavorable bulky classic Hodgkin lymphoma (cHL) is a combination of chemotherapy and radiotherapy. However, radiotherapy is associated with long-term consequences and toxicities. The CALGB-50604 ALLIANCE trial (NCT01132807) has previously demonstrated that four cycles of chemotherapy consisting of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) show durable progression-free survival (PFS) for a majority of patients with early-stage, nonbulky HL and an interim negative positron emission tomography (PET) scan. At the 2021 ASCO Annual Meeting, Ann S. LaCasce1 presented the findings from the CALGB-50801 ALLIANCE study (NCT01118026) evaluating the efficacy and safety of response-adapted therapy based on PET in patients with bulky stage I and II HL. The hypothesis was that PET-negative patients do not require radiotherapy, whilst PET-positive patients will benefit from escalation to BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) + radiotherapy. The key findings are summarized below.
Study design
A single-arm, phase II clinical trial of response-adapted PET therapy in patients aged ≥18 years, with stage IA−IIB cHL, Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0−2, and bulky disease >10 cm or mediastinal mass > 0.33 maximal intrathoracic diameter on chest x-ray. Eligible patients who had received PET following two cycles of chemotherapy (PET2), received treatment as shown in Figure 1. PET-negative was defined as Deauville 1−3 and PET-positive as Deauville 4−5.
Figure 1. Treatment schema*
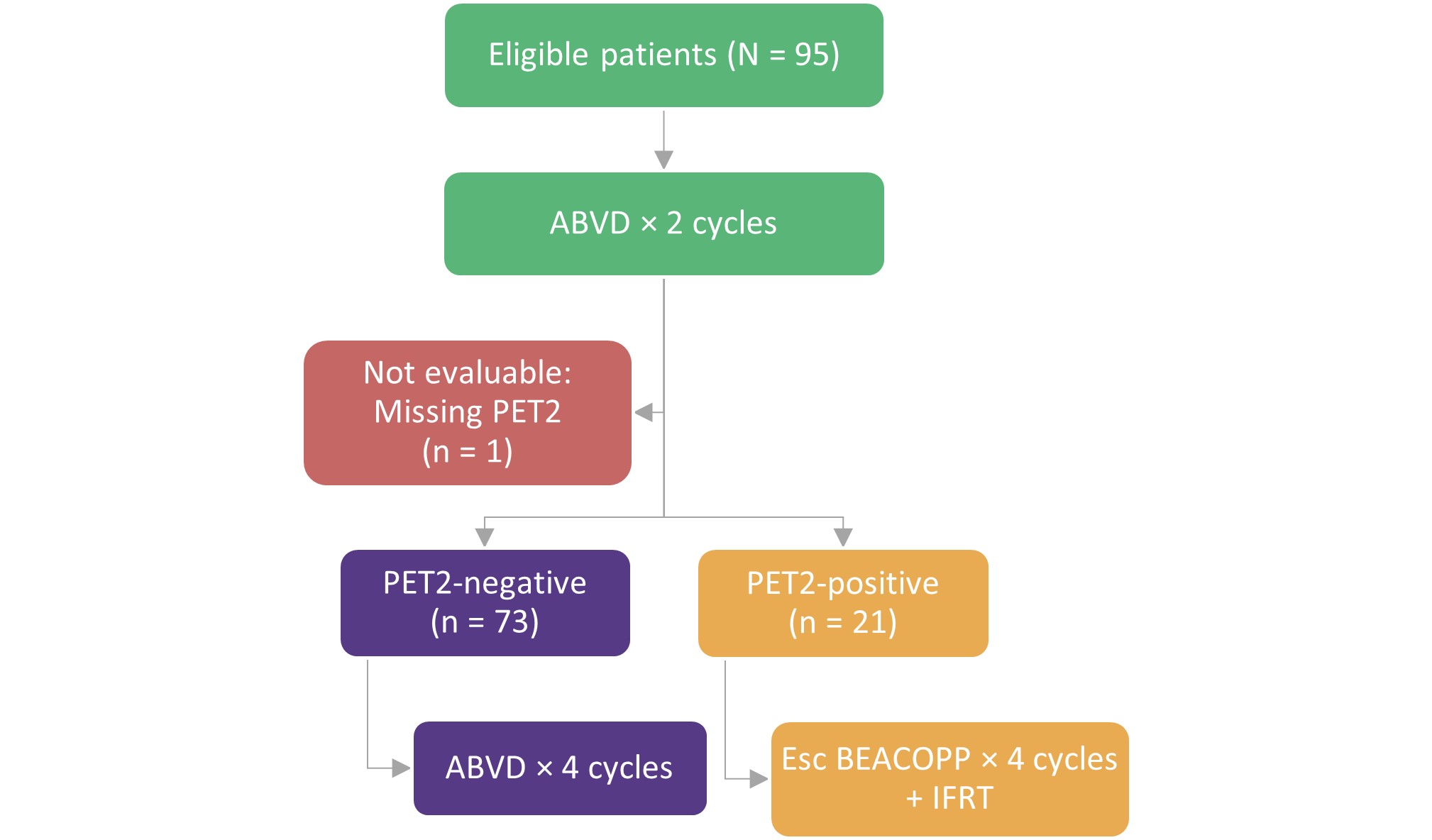
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; Esc BEACOPP, escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; IFRT, involved-field radiation therapy; PET2, positron emission tomography following two cycles of chemotherapy.
*Adapted from LaCasce et al.1
- The primary endpoint was PFS, defined as the time from study enrollment to disease progression or death. Secondary endpoints included pulmonary toxicity and hematologic/infectious toxicities.
- The primary endpoint was met when hazard ratio (HR) for PET2-positive vs PET2-negative was <4.1 (one-sided; p = 0.04).
Baseline characteristics
Median age of patients was 30 years (range, 18−58) and included 53% women. 78% of patients were PET2-negative, and majority of the patients had stage II disease (Table 1).
Table 1. Baseline characteristics*
|
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ECOG-PS, Eastern Cooperative Oncology Group performance status; PET2, positron emission tomography following two cycles of chemotherapy. |
||||
|
Characteristic |
Total |
PET2-negative |
PET2-positive |
p value |
|---|---|---|---|---|
|
Sex, Female, % |
53 |
56 |
43 |
0.33 |
|
Age, median (range) |
30 (18−58) |
30 (18−58) |
28 (19−56) |
0.39 |
|
Stage, % |
0.78 |
|||
|
IA/IAE |
7 |
8 |
5 |
— |
|
IB |
2 |
3 |
0 |
— |
|
IIA/IIAE |
39 |
41 |
33 |
— |
|
IIB/IIBE |
51 |
48 |
62 |
— |
|
ECOG-PS, % |
0.14 |
|||
|
0 |
68 |
71 |
57 |
— |
|
1 |
31 |
29 |
38 |
— |
|
2 |
1 |
0 |
5 |
— |
|
Prior ABVD, % |
16 |
18 |
10 |
0.51 |
Results
Efficacy
- Median follow-up was 58.4 months (range, 0−117.4 months).
- Primary endpoint for PFS was met (HR = 1.03; 85% upper confidence bound = 2.38).
- At 3 years, the estimated PFS was 90% (95% CI, 77−100) and 93% (95% CI, 87−99) in PET2-positive and PET2-negative patients, respectively.
- At a median follow-up of 66.2 months (range, 2.9−117.4 months), the estimated overall survival was 94% (95% CI, 84−100) and 99% (95% CI, 96−100) in PET2-positive and PET2-negative patients (HR = 1.2, 95% CI, 0.12−11.60), respectively.
- One patient died in the PET2-positive arm, due to lymphoma and pneumonia, and three patients died in the PET2-negative arm due to lymphoma, anaplastic astrocytoma, and chronic obstructive pulmonary disease.
Safety
- All six cycles of bleomycin were received in 70% and 76% of PET2-negative and PET2-positive patients, respectively.
- Grade ≥3 pulmonary toxicities occurred in 8% of the PET2-negative patients; none occurred in the PET2-positive patients (Table 2).
- Grade 4 neutropenia was as expected in PET2-negative patients, at 74%, compared to 57% in PET2-positive patients.
- PET2-negative patients had very few occurrences of thrombocytopenia and no occurrence of sepsis.
Table 2. Adverse events*
|
PET2, positron emission tomography following two cycles of chemotherapy. |
|||
|
Adverse event, % |
Any grade |
Grade 3 |
Grade 4 |
|---|---|---|---|
|
Pulmonary: PET2-negative† |
|||
|
Cough |
62 |
3 |
0 |
|
Dyspnea |
58 |
3 |
0 |
|
Hypoxia |
1 |
1 |
0 |
|
Pneumonitis |
4 |
1 |
0 |
|
Pulmonary: PET2-positive‡ |
|||
|
Cough |
62 |
0 |
0 |
|
Dyspnea |
33 |
0 |
0 |
|
Hypoxia |
0 |
0 |
0 |
|
Pneumonitis |
0 |
0 |
0 |
|
Hematologic/infectious: PET2-negative |
|||
|
Neutropenia |
93 |
12 |
74 |
|
Thrombocytopenia |
8 |
1 |
1 |
|
Febrile neutropenia |
8 |
8 |
0 |
|
Sepsis |
0 |
0 |
0 |
|
Hematologic/infectious: PET2-positive |
|||
|
Neutropenia |
100 |
29 |
57 |
|
Thrombocytopenia |
71 |
14 |
14 |
|
Febrile neutropenia |
10 |
10 |
0 |
|
Sepsis |
5 |
0 |
5 |
Conclusion
This first prospective study restricted to patients with bulky stage I/II cHL, demonstrated excellent PFS in all patients treated with PET-adapted therapy, including 78% of PET-negative patients for which radiotherapy or exposure to escalated BEACOPP was not required. Based on these results the investigators recommend omitting radiotherapy in PET2-negative patients who receive six cycles of ABVD. However, the study was limited by a small number of PET2-positive patients and a nonrandomized study design. Further studies comparing the efficacy, safety, and cost of novel agents with PET-adapted therapy are suggested.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

