All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Incyte, and Lilly. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
AACR 2017 | Overexpression of PD-L1 contributes to immune evasion of Adult T-Cell Leukemia/Lymphoma
On Monday 3rd April at AACR 2017, during the “SY16 - Mechanisms Regulating Immune Checkpoint Therapies” session, Seishi Ogawa of Kyoto University Graduate School of Medicine, Kyoto-shi Sakyo-ku, Japan, gave a talk titled “A novel genetic mechanism of evading antitumor immunity in multiple human cancers.”
The talk began by explaining that tumor cells utilize PD-L1 signaling to escape from the host’s immune response; immunotherapy resulting in PD-L1 blockade prevents this occurring.

Antibodies against PD-1/PD-L1 include nivolumab and pembrolizumab and can be used against a variety of cancer types including lung, kidney, ovarian, colorectal, and head & neck cancers, as well as melanoma and Hodgkin Lymphoma. In those that do respond, anti-PD-1/PD-L1 antibodies achieve durable responses. However, ORR is only around 20–30% and super responders are rare (1–2%). Organ damage (sometimes life-threatening) can occur because of auto-immunity and there are currently no reliable markers for predicting response.
Seishi Ogawa then focused the talk of Adult T-Cell Leukemia/Lymphoma (ATL):

There is a long latency period before the onset of ATL. Infection with HTLV-1 is not enough to develop ATL; genetic alterations in cellular and/or viral genes are required.
During genetic analysis of ATL samples, a novel breakpoint cluster was identified at the PD-L1 locus (9p24.1). This area is affected by a range of Structural Variants (SVs) which often result in the 3’ UTR being disrupted; often truncating the 3’ UTR. In SV+ ATL samples, PD-L1 expression has been found to be increased.
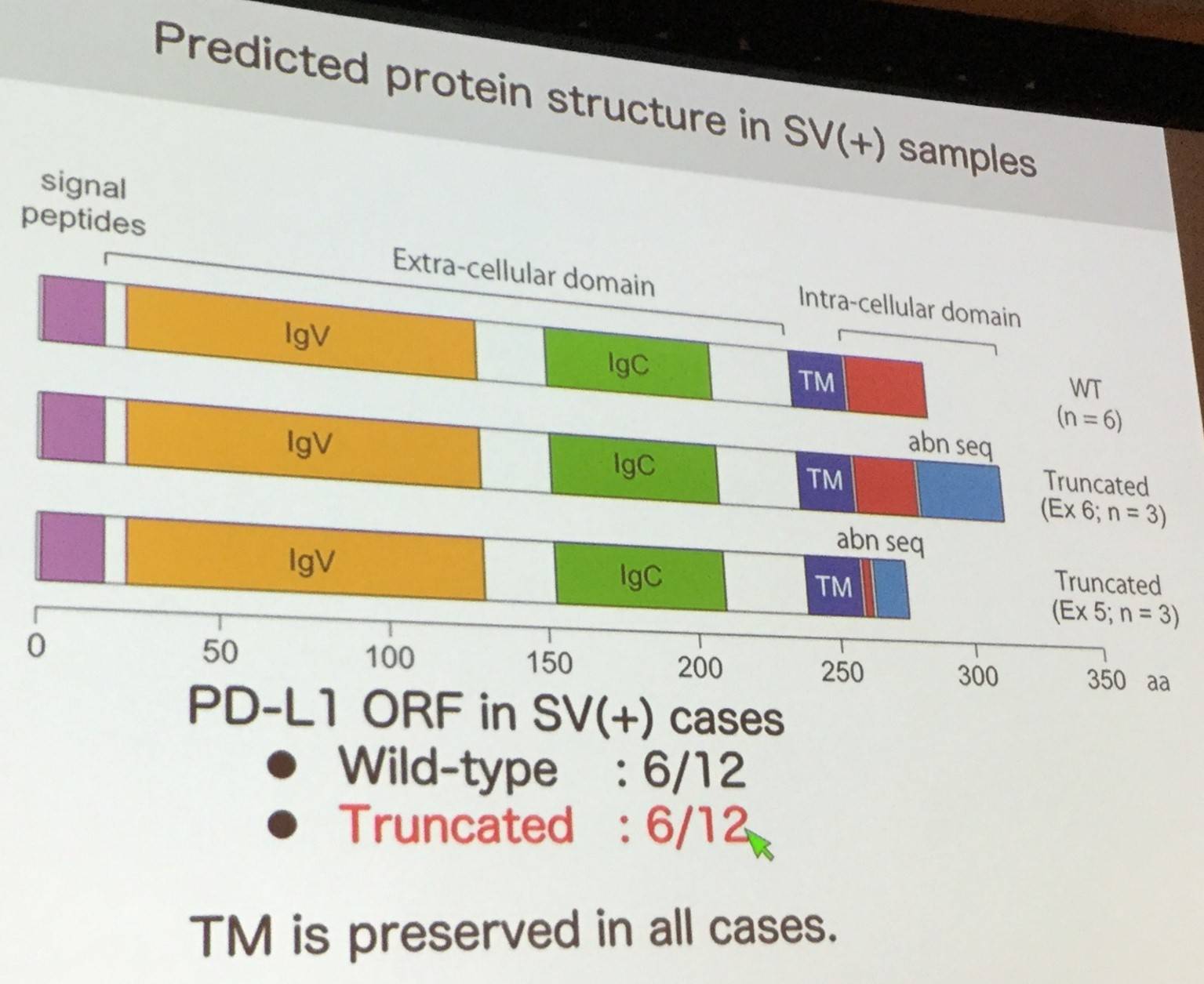
Moreover, surface expression and PD-1 binding ability is preserved in aberrantly expressed PD-L1, and experiments disrupting the 3’ UTR resulted in stabilization of PD-L1 transcripts.

In other cancers, such as Lung Adenocarcinoma and Skin Squamous Cell Cancer, as PD-L1 expression increases, anti-tumor response (cytolytic activity) decreases.

Moreover, Ogawa presented data of a mouse SV+ thymoma model which demonstrated that cancer cells escape the immune response, however administration of αPd-l1 antibody disrupts immune evasion resulting in a decrease in tumor volume. Other slides were also presented, showing data that disruption of PD-L2 3’ UTR also increases PD-L2 expression.
The talk was concluded with a succinct summary slide, and Ogawa emphasized the unexpected role of 3’ UTR regulation in expression of PD-L1 and PD-L2. Screening for SVs involving 3’ UTRs could potentially identify patients whose cancers actively evade their immune response, and could become a novel marker for predicting patients’ responses to therapy using immune checkpoint blockade.

References

