All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Advanced-stage cHL International Prognostication Index (A-HIPI): A modern clinical prediction model from the HoLISTIC consortium
Classic Hodgkin lymphoma (cHL) is a B-cell malignancy with favorable disease outcomes; however, it lacks single consensus-based or personalized treatment strategies beyond multiagent chemotherapy. For the past 25 years, the International Prognostic Score (IPS), developed by the German Hodgkin Study Group in 1998, has been widely used as a prognostic tool for progression-free survival (PFS) and overall survival (OS) in cHL. The IPS-7 consisted of seven clinical factors for survival and used dichotomous categorization of patient and disease factors with limited information on discrimination and calibration. Furthermore, the IPS-7 was based on complete case analyses, where missing data were frequent and required imputation strategies that were not externally validated.1
Poor calibration of IPS-7 among contemporarily treated patients and advancements in management strategies and outcomes for cHL calls for a need to update and refine the existing prognostic tools.1
To address this unmet need, the Hodgkin Lymphoma International Study for Individual Care (HoLISTIC) consortium was formed in 2018 to develop and validate a modern clinical prediction model for cHL. Rodday et al.1 recently published an article in Journal of Clinical Oncology describing the development and validation of the Advanced-stage cHL International Prognostication Index (A-HIPI) to predict 5-year PFS and OS in adult patients with untreated, advanced-stage cHL.1 Herein, we summarize the key findings.
Methods1
A multivariable prediction model was developed using individual patient data obtained from the following phase III international clinical trials in newly diagnosed cHL, conducted from 1996 to 2014:
- RATHL (n = 933)
- ECOG2496 (n = 632)
- HD0607 (n = 769)
- HD0801 (n = 466)
- SW0G0816 (n = 322)
- UK Stanford V (n = 314)
- HD9601 (n = 310)
- HD2000 (n = 276)
The model was then validated in a separate cohort of patients enrolled in the following real-world cHL registries across North America:
- British Columbia Cancer (n = 602)
- Princess Margaret Hospital (n = 318)
- Australasian Lymphoma and Related Diseases Registry (n = 260)
- Iowa/Mayo SPORE (n = 251)
Patients aged 18–65 years with stage IIB, III, or IV disease were included in the study. Patients with missing data on more than 50% of the candidate predictor variables were excluded. Multiple imputation was used for missing data.
The primary outcomes were 5-year PFS and OS. Baseline predictors were sex, stage (IIB, III, IV), B symptoms, any bulk (by trial/registry definition), histology, age at diagnosis, white blood cell count, absolute lymphocyte count (ALC), hemoglobin, albumin, and erythrocyte sedimentation rate.
Piecewise linear splines were evaluated for potential nonlinear relationships over a full range of continuous variables. Cox hazard models were used to estimate 5-year PFS and OS. The model was evaluated in the external validation cohort and compared using Harrell’s C-statistics to assess discrimination. Observed and predicted probabilities of 5-year outcomes were compared to assess calibration. The performance of IPS-7 and IPS-3 were also examined in the validation cohort.
Results1
A total of 4,022 patients were included in the model development cohort and 1,431 were included in the validation cohort, with a median follow-up of 62 months (interquartile range [IQR], 36−90) and 74 months (IQR, 31−132), respectively. The baseline patient and disease characteristics are presented in Table 1.
Table 1. Baseline patient characteristics*
|
ESR, erythrocyte sedimentation rate; NOS, not otherwise specified; SD, standard deviation; WBC, white blood cell. |
||
|
Characteristic, % (unless otherwise stated) |
Development |
Validation |
|---|---|---|
|
Mean age (SD), years |
35.1 (12.0) |
35.6 (13.1) |
|
Female |
45.5 |
43.5 |
|
Stage |
||
|
Stage IIB |
27.5 |
37.5 |
|
Stage III |
39.0 |
29.8 |
|
Stage IV |
33.5 |
32.7 |
|
Histology |
||
|
Lymphocyte depleted |
1.1 |
0.5 |
|
Lymphocyte rich |
2.5 |
1.5 |
|
Mixed cellularity |
13.0 |
5.9 |
|
Nodular sclerosis |
74.2 |
71.5 |
|
NOS |
9.1 |
20.6 |
|
B symptoms |
73.1 |
77.1 |
|
Any bulk |
35 |
30.3 |
|
Mean WBC count (SD), 103/µL |
10.7 (5.3) |
10.8 (5.2) |
|
Categorical lymphocyte count, 103/µL |
||
|
0.1–2 |
79.1 |
81.0 |
|
2–5 |
20.9 |
19.0 |
|
Mean hemoglobin (SD), g/dL |
12.0 (1.9) |
12.0 (1.9) |
|
Mean albumin (SD), g/dL |
3.7 (0.6) |
3.7 (0.6) |
|
Mean ESR (SD), mm/h |
59.0 (35.7) |
52.8 (35.6) |
Kaplan-Meier estimates were 77% (95% confidence interval [CI], 76%–78%) for 5-year PFS and 92% (95% CI, 91%–93%) for 5-year OS.
Piecewise linear splines yielded non-linear, U-shaped relationships between continuous variables (age, ALC, white blood cell count, hemoglobin, albumin, and erythrocyte sedimentation rate) and survival outcomes (PFS and OS). The model variables with parameter estimates, HRs, and the optimism-corrected beta coefficient on multivariable analyses for the A-HIPI model are delineated in Figure 1. The effect estimates represented a 1-unit increase in the predictor variable. Age, stage, albumin, and ALC were significant for both 5-year PFS and OS; while sex, hemoglobin, and bulk disease were significant only for 5-year OS.
Figure 1. Optimism-corrected HRs for A 5-year PFS and B 5-year OS multivariable prediction models*
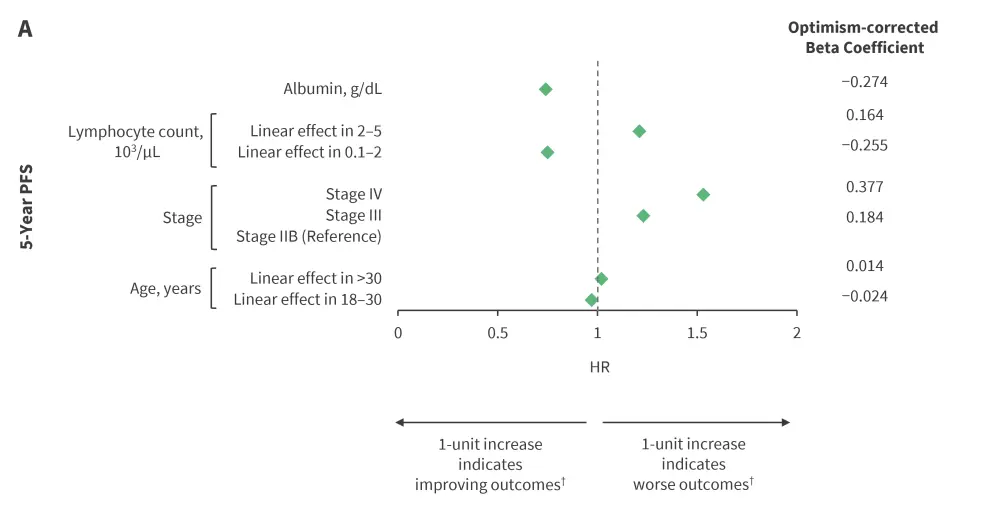
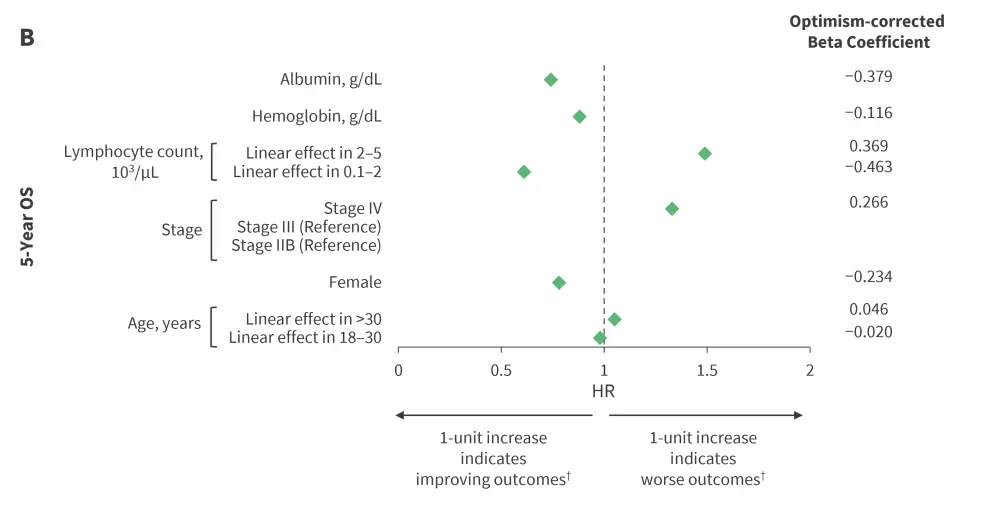
HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
*Data from Rodday, et al.1
†Piecewise linear splines are interpreted as follows: 1-year increase in age indicated improving outcomes in patients aged 18–30 years and worse outcomes in those older than 30 years. Likewise, 1-unit increase in lymphocyte count indicated improving outcomes for an ALC of 0.1–2.0 (103/mL) and worse outcomes for an ALC 2.0–5.0 (103/mL).
Table 2. Comparison of c-statistic for A-HIPI and the IPS-7 and IPS-3*
| C-statistics | A-HIPI | IPS-7 | IPS-3 | |
|---|---|---|---|---|
| 5-year PFS | Development cohort | 0.595† | 0.583 | 0.566 |
| Validation cohort | 0.590 | 0.597 | 0.579 | |
| 5-year OS | Development cohort | 0.717† | 0.663 | 0.658 |
| Validation cohort | 0.730 | 0.692 | 0.657 | |
|
A-HIPI, Advanced-stage Hodgkin lymphoma International Prognostication Index; IPS, International Prognostic Score; OS, overall survival; PFS, progression-free survival. |
||||
Kaplan-Meier observed PFS and OS rates stratified by quartile of predicted risk in the external validation cohort ranged from 71.7% to 86.9% and 82.9% to 96.5%, respectively.
In the external validation cohort, A-HIPI showed good calibration except for a slight overestimation of risk in the highest risk decile, whereas IPS-7 showed an overestimation of risk among all risk groups. The calibration intercept and slope were −0.40 (95% CI, −0.82 to 0.00) and 0.83 (95% CI, 0.50 to 1.16), respectively, for A-HIPI PFS model and −0.43 (95% CI, −0.96 to 0.09) and 0.89 (95% CI, 0.66 to 1.11), respectively, for A-HIPI OS model.
Conclusion1
Rodday et al.1 developed and externally validated a modern-day A-HIPI model in newly diagnosed patients with advanced-stage cHL from recent seminal clinical trials and cHL registries. They identified unique prognostic variables significant for PFS versus OS, including non-linear relationships between continuous variables and patient outcomes. Additionally, the A-HIPI exhibited superior discrimination for OS and enhanced calibration for both PFS and OS compared with the historic IPS.
To enhance the application of the A-HIPI model, an online calculator has been created to assist physicians and patients with individualized prognostication.
Further studies are warranted to include the wider age spectrum, especially adults older than 65 years. These data are also needed to be validated in patients receiving immediate intensive chemotherapy or frontline novel targeted therapies. Furthermore, simulation modeling is needed to estimate the personalized risk of post-acute and late effects based on patient and treatment-related variables.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

