All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Educational Session: Novel Agents and Strategies in Relapsed/Refractory Hodgkin Lymphoma
Bookmark this article
On Saturday 3rd December, during the 58th Annual Meeting & Exposition of the American Society of Hematology (ASH), in San Diego, CA, an educational session took place between 9.30am–11.00am, titled “Hodgkin Lymphoma: Treatment Today and the Challenge for Tomorrow.” This session was Chaired by Andrew Lister, MD, of Barts Cancer Institute, London, UK, and focused on how the outcome of patients with HL in 2016 can be improved, beyond that achieved in the last 50 years, with combination chemotherapy and extended-field megavoltage radiotherapy.
“Novel agents and strategies in transplant-eligible patients with relapsed and refractory Hodgkin Lymphoma” was the second talk during this session, and was presented by Craig H. Moskowitz, MD, from the Memorial Sloan Kettering Cancer Center.
Dr Moskowitz began his talk by explaining that out of 8,000 patients with HL, treatment will fail in approximately 1,400 patients.
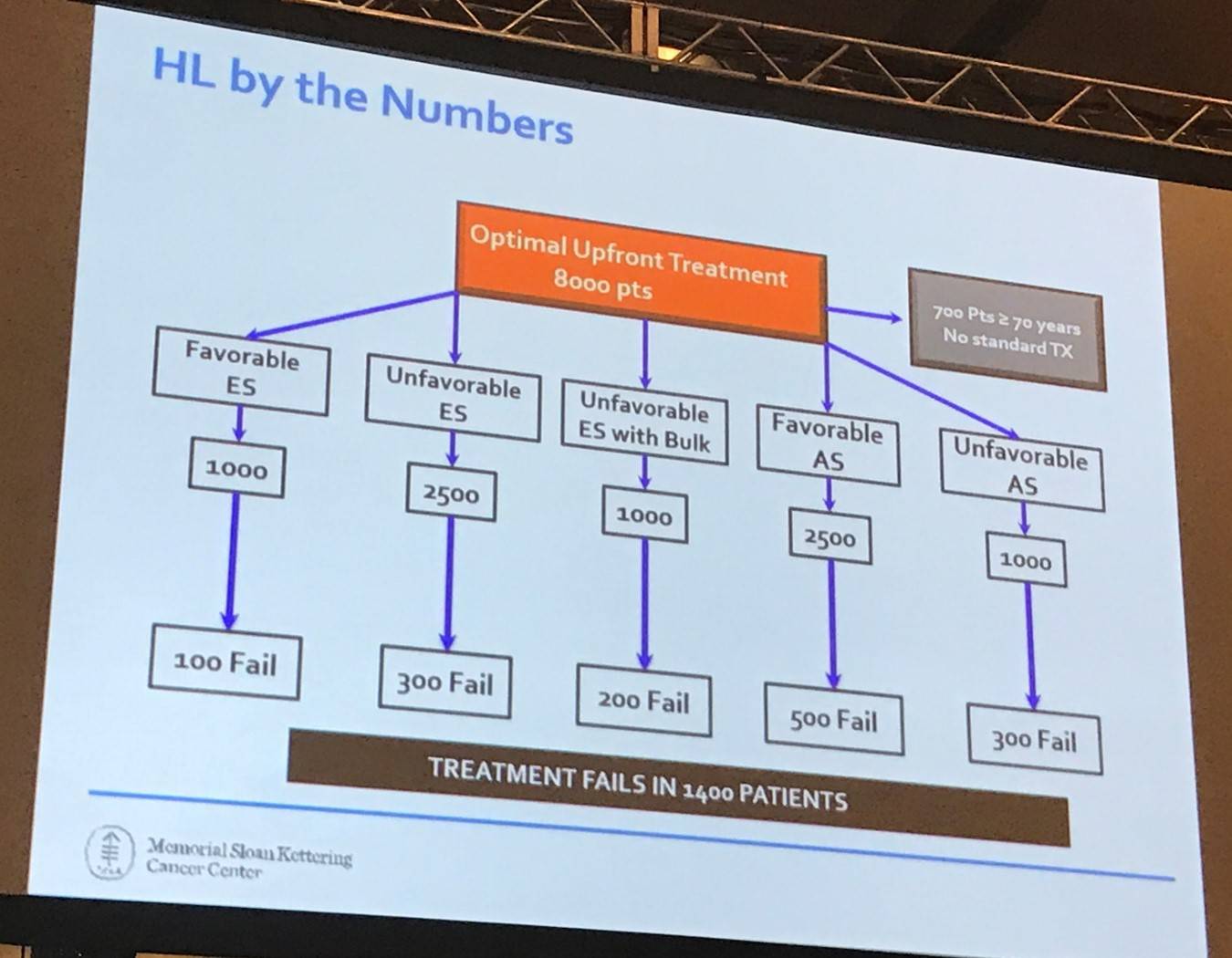
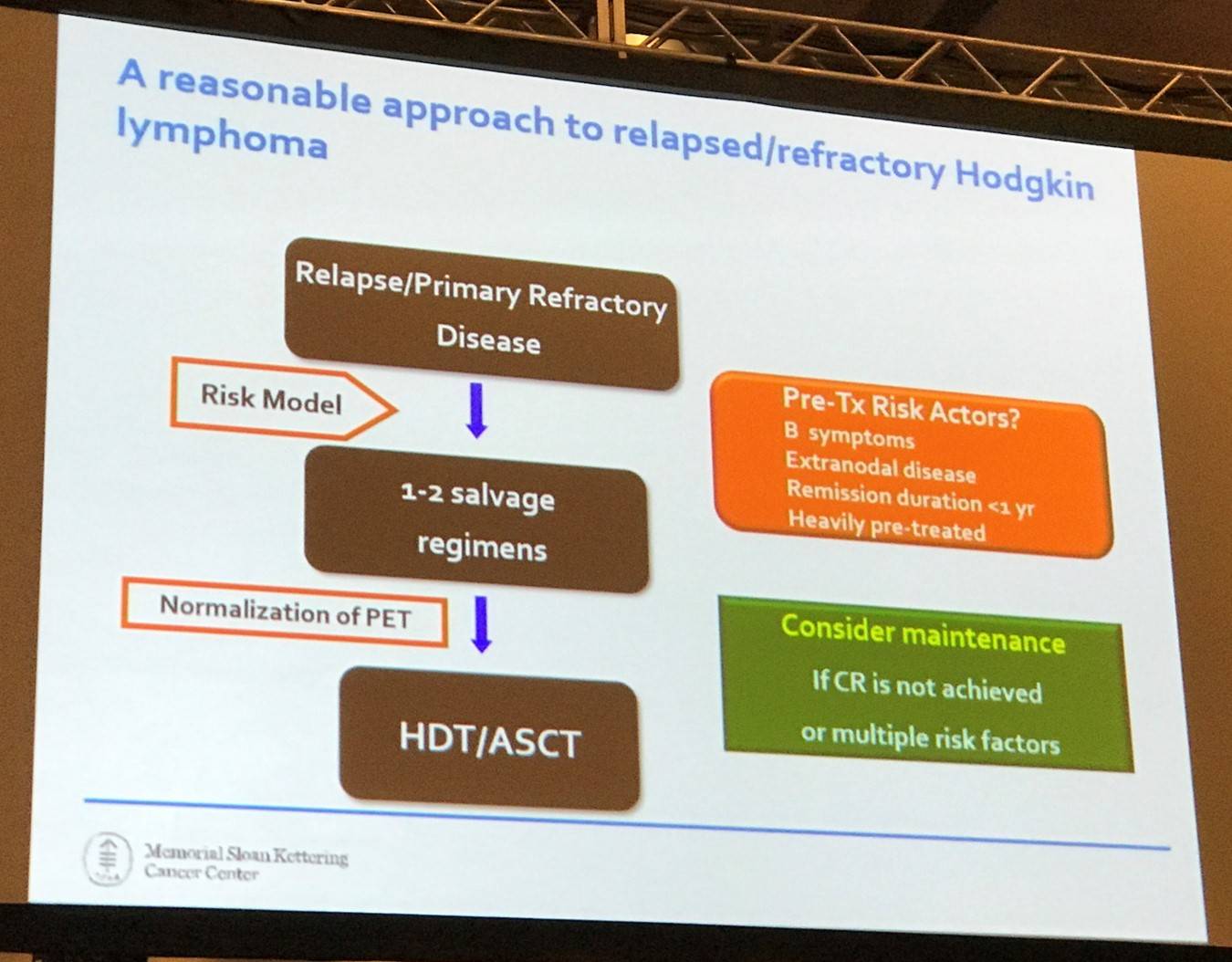
A potential approach to treating R/R HL was then presented, however there are some issues with this:
- Who should get post-ASCT maintenance therapy?
- Why is pre-ASCT PET status so important?
- Should BV be administered as part of salvage therapy?
- How will the checkpoint inhibitors be used?
- Is there a home for allogeneic stem cell transplantation?
Dr Moskowitz then made the point that the randomized studies we are conducting and writing now will not be reported for another 5 years. Drug development is moving very rapidly and so is approval of new agents; the era of the phase I-II-III paradigm needs to end. Phase III trials may have little impact on how patients are treated, a lesson learned from the AETHERA study.
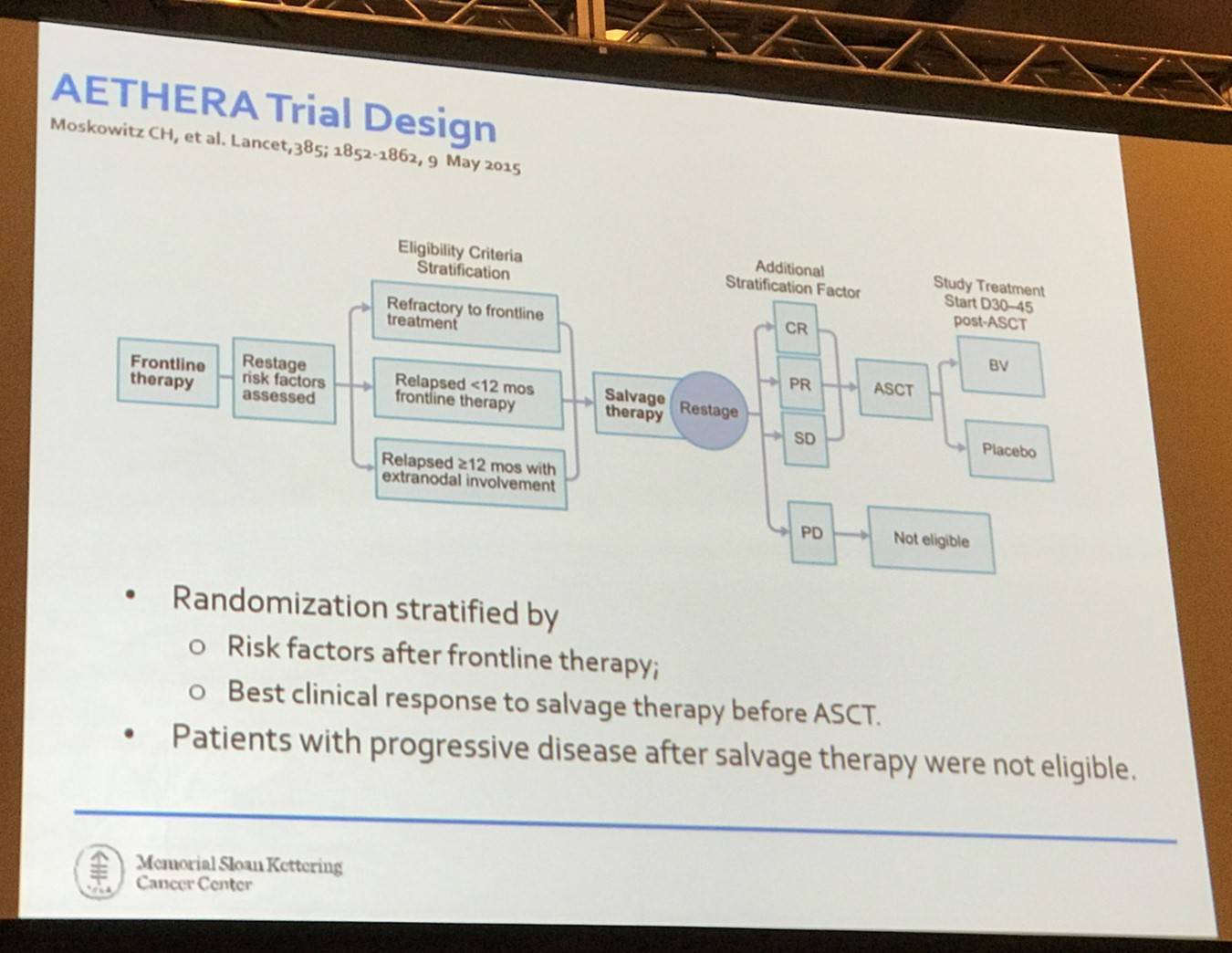
In this study, only 10% of patients had one unfavorable prognostic factor of the five factors predicted for PFS:
- Initial remission duration <1 year
- <CR to most recent salvage therapy
- Extranodal disease at pre-ASCT relapse
- B-symptoms at pre-ASCT relapse
- >1 ST required to achieve chemosensitive disease
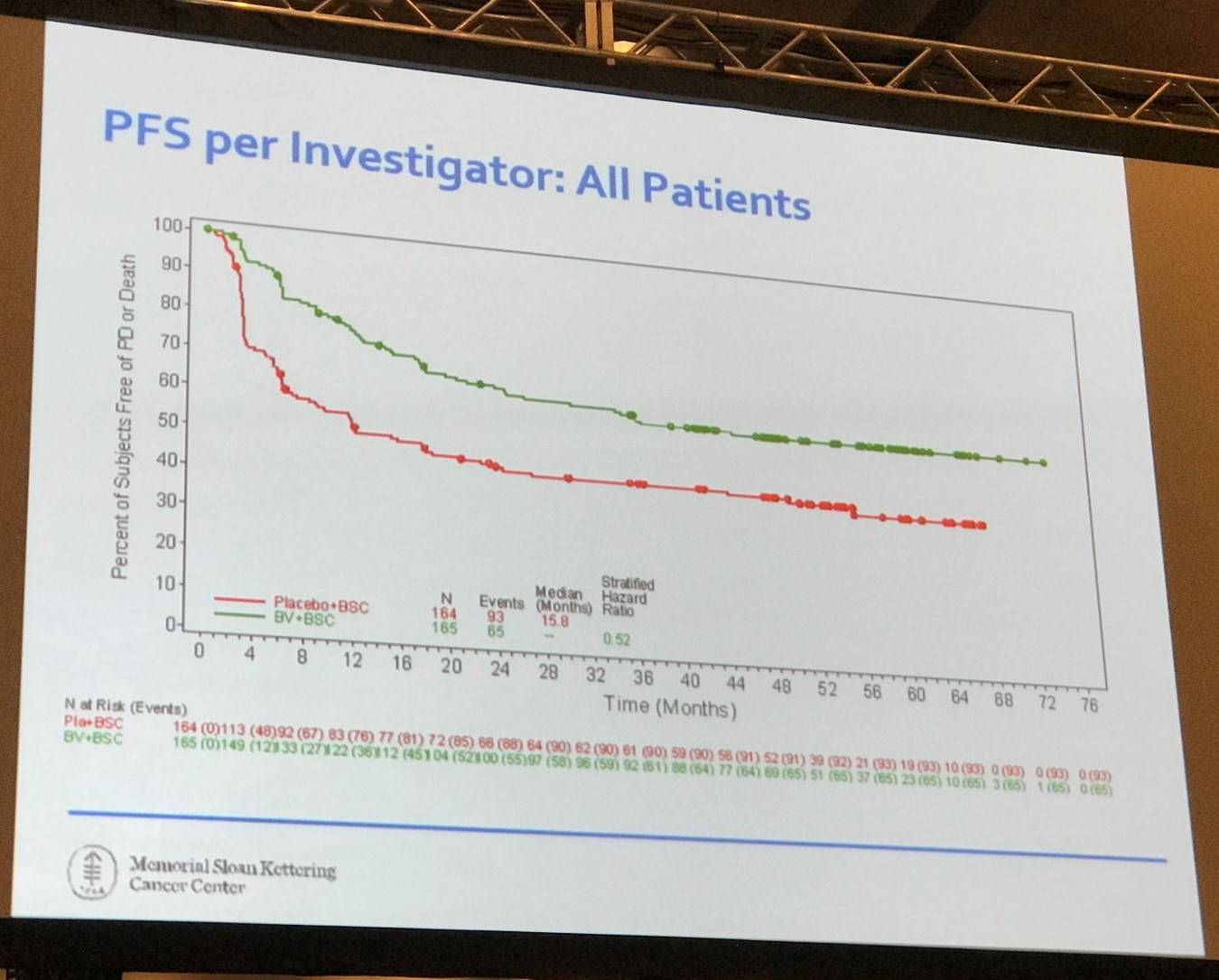
There are a number of issues concerning the AETHERA study:
- Neuropathy: 90% resolution to grade 1 or less; remember to dose reduce if grade II
- Overall Survival: New era with CPI (checkpoint inhibitors); only time will tell if there will be a difference, but indefinite palliative therapy is not effective or efficient and does not provide a good QoL for patients
- Pre-ASCT PET outcome:
- Only 1 of 5 risk factors in this study
- Not prospectively done, read centrally or defined by Deauville criteria
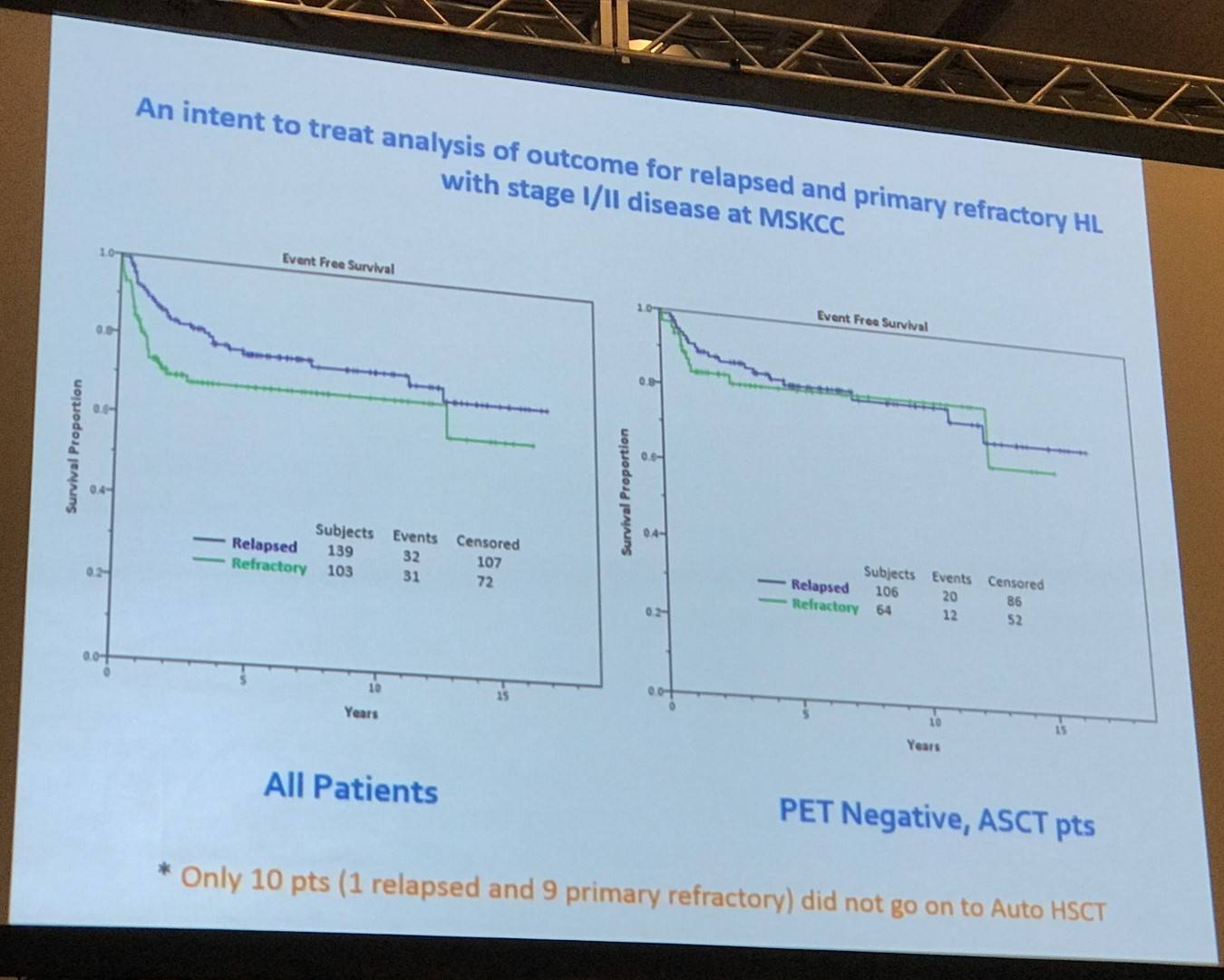
The two most important issues when evaluating patients for ASCT were then outlined:
- Is there stage IV disease pre-salvage therapy?
- Is the patient in CR, i.e. PET negative post-salvage therapy?
Between 1994 and 2003, second-line therapy was risk-adapted based on the MSKCC 3 factor model: B-symptoms, extranodal disease, and relapse <1 year. Pre-transplant functional imaging was the most significant determinant of outcome. Currently, PET is used pre-ASCT and it has been shown to be consistently prognostic.
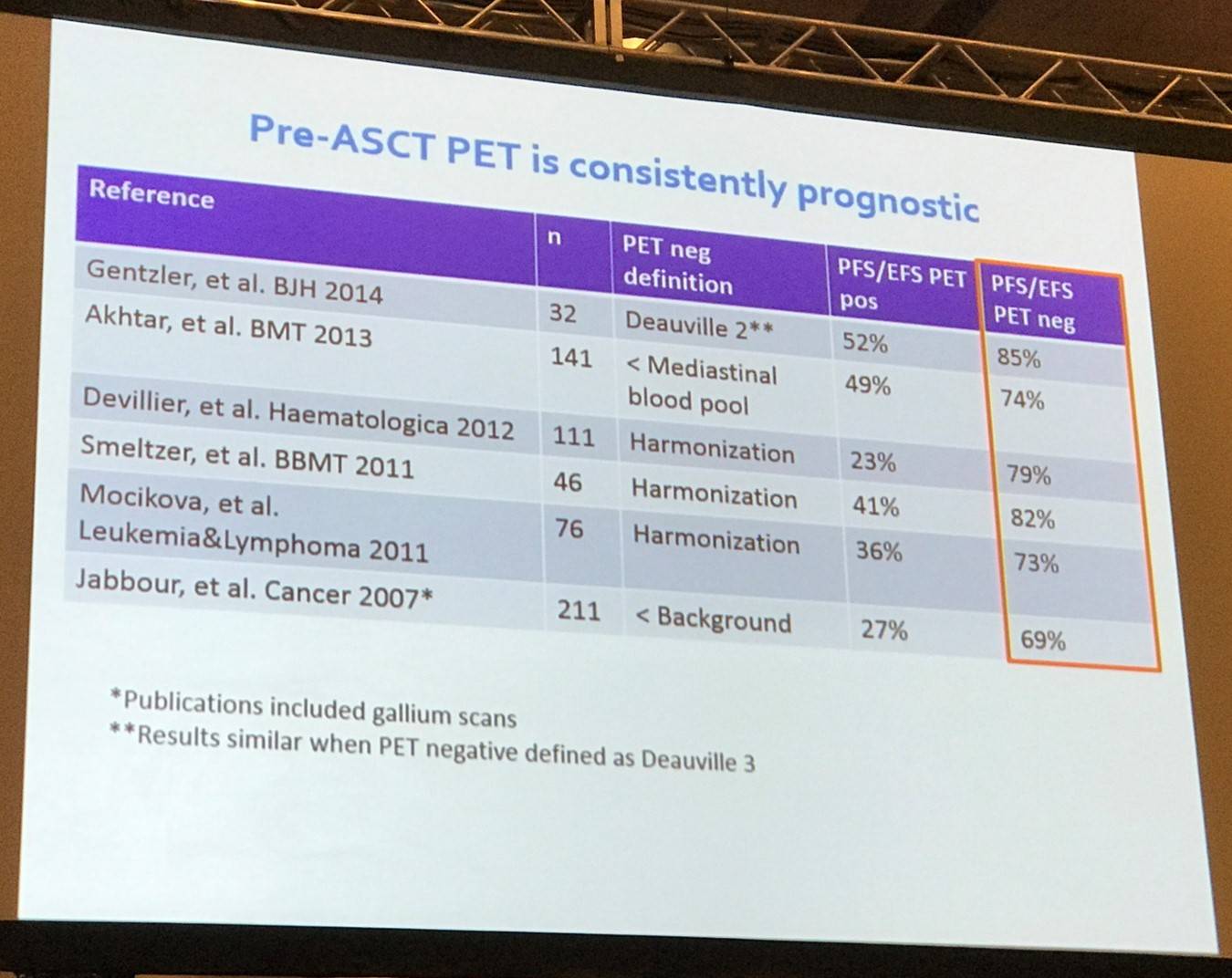
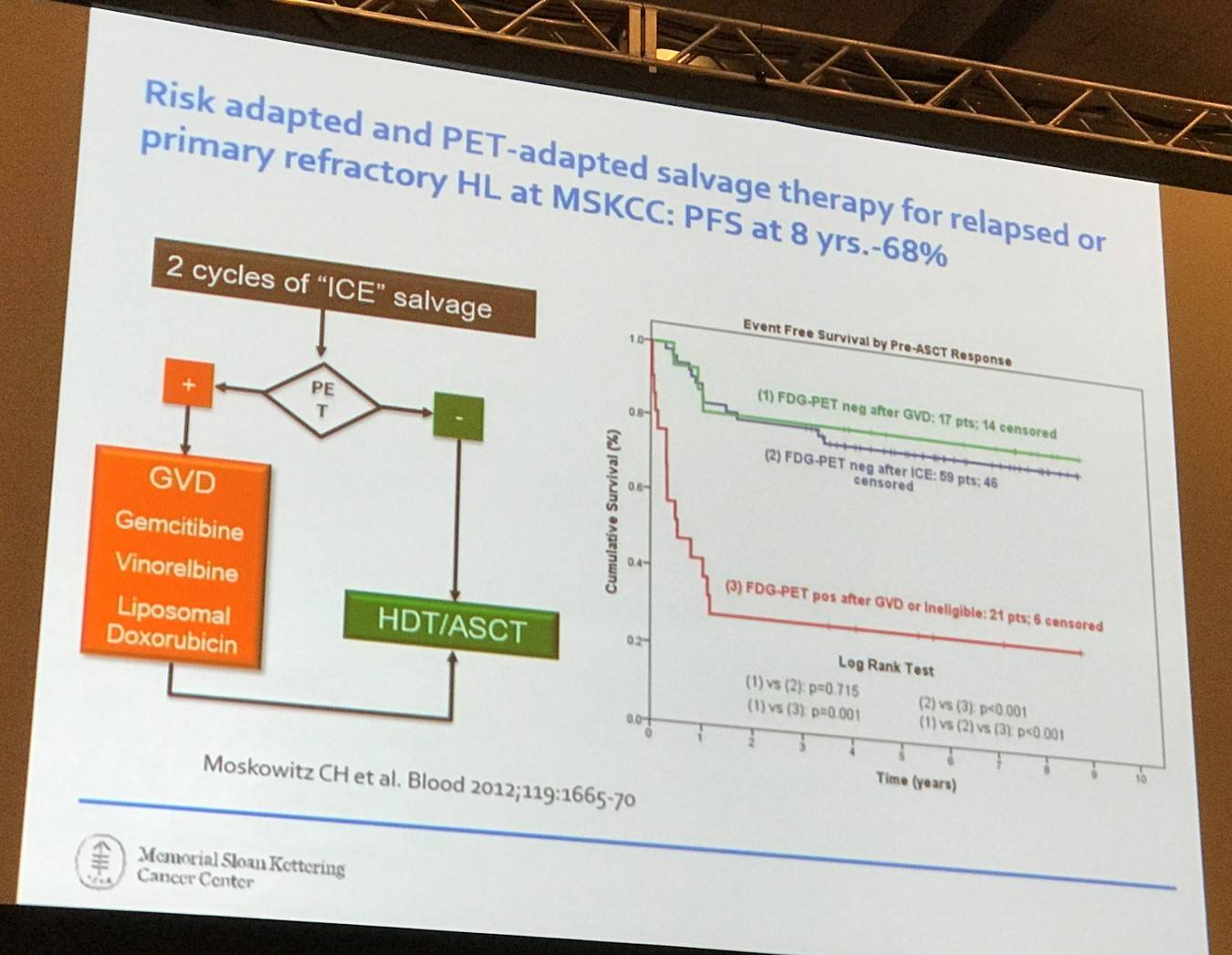
The next topic explored in this talk was salvage therapy, beginning with an overview of the current status:
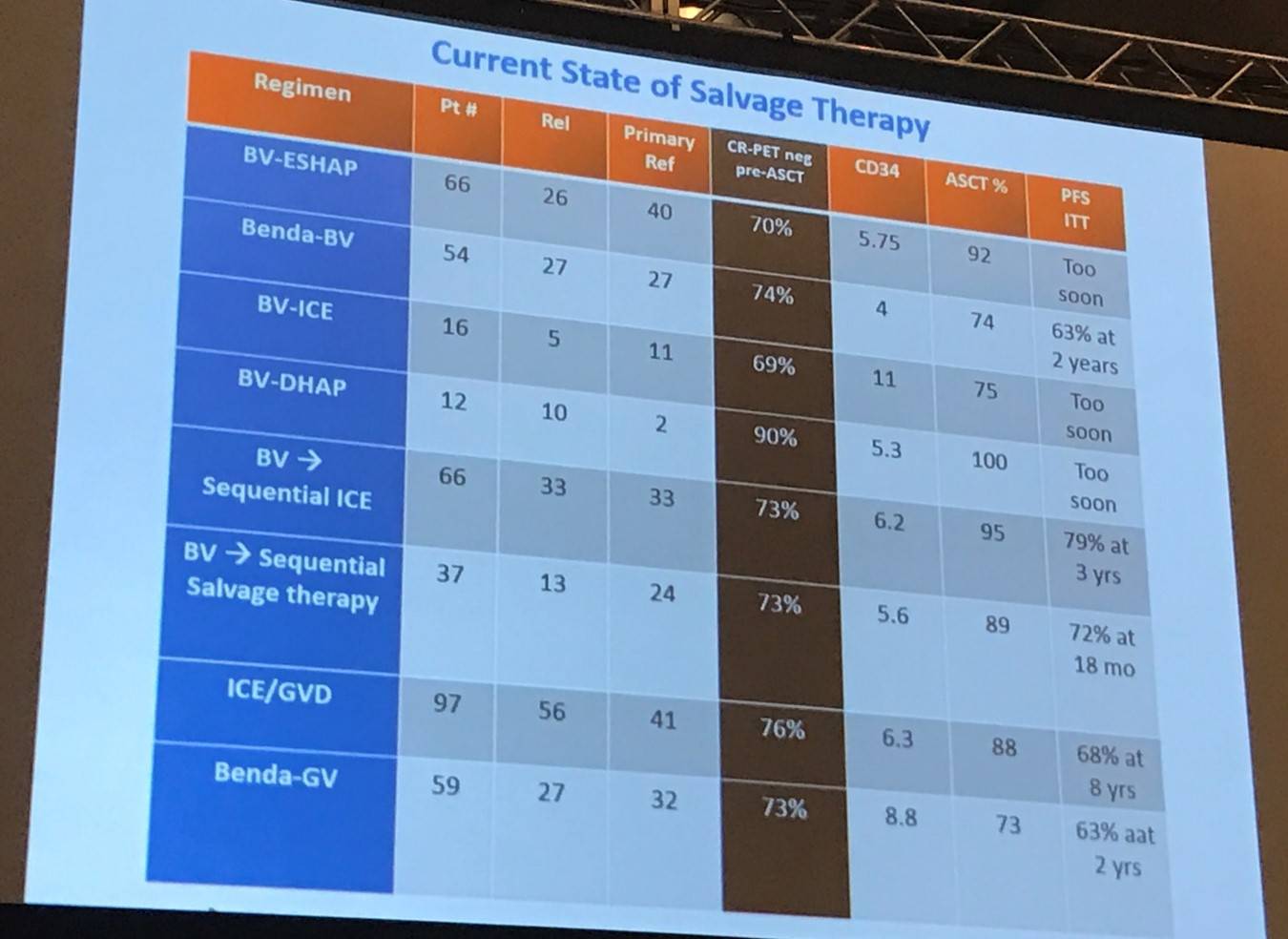
Dr Moskowitz went on to recommend sequential therapy, and also explained why:
- There is no evidence that a CR to single agent BV is inferior to that of chemotherapy or chemo-immunotherapy
- 1/3 of patients can avoid chemotherapy for salvage if BV is used first
- Chemotherapy alone without BV offers a CR rate of 60–73% with ICE or BeGV
- BV can be used as salvage number 2
- Bendamustine-BV seems no better than BeGV
- Platinum based salvage regimens combined with BV are “challenging”
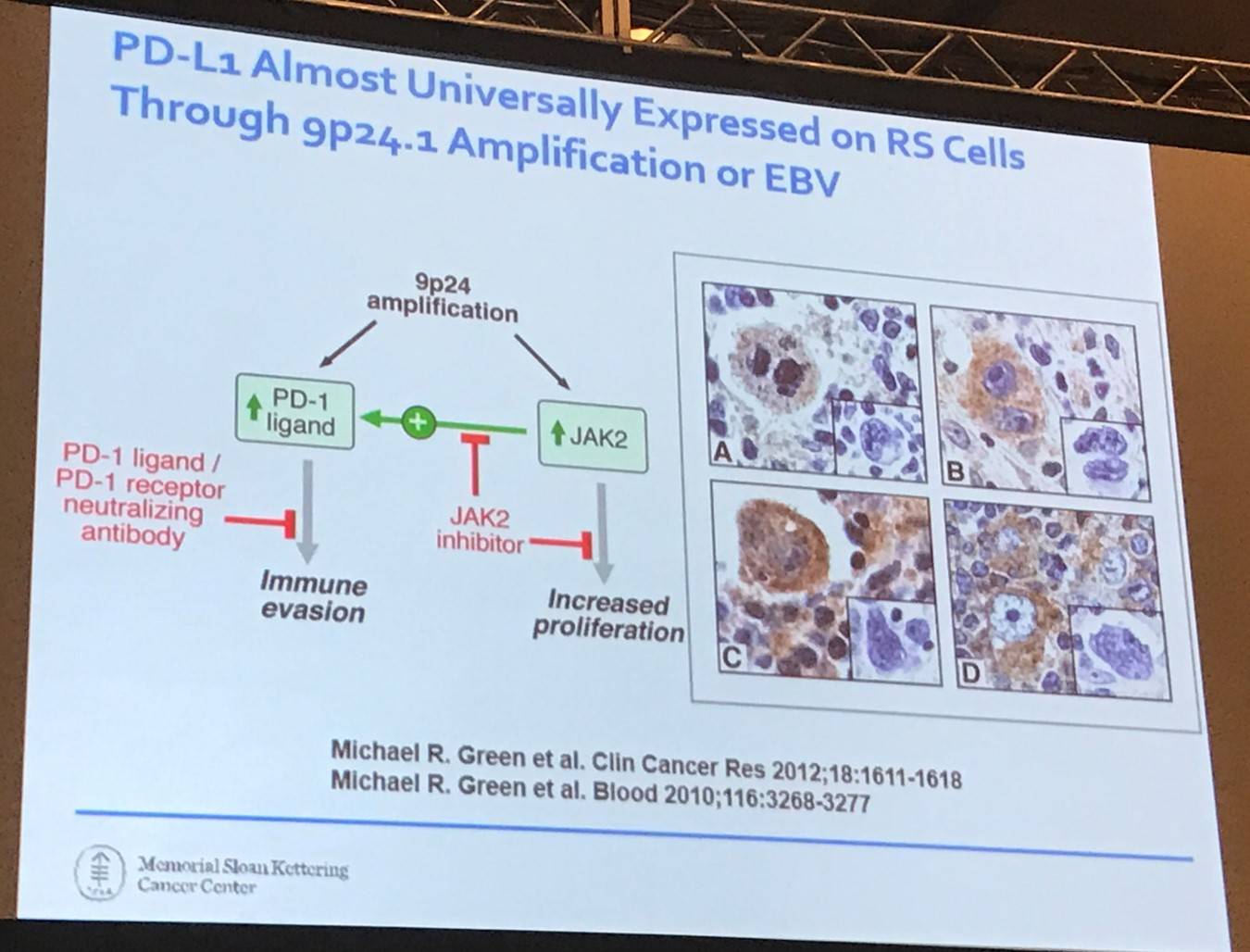
The talk then focused on checkpoint inhibitors, specifically PD-1 blockade. Currently, there are 75 prospective clinical trials open at MSKCC studying checkpoint inhibitors in solid and liquid tumors. In terms of mechanism, currently we know that PD-1 blockade releases inhibition on adaptive immunity in solid tumors:
- PD-1 ligand expression is predictive of response
- Anti-tumor immunity is mediated by CD8+ effector T-cells
- Enhanced by increased tumor mutation burden
- Requires MHC-I
However, is this how it works in HL? Probably not:
- CD8+ T-cells are not prevalent in the HL microenvironment
- Frequent loss of MHC-I prevents recognition by CD8+ T-cells
- Frequent loss of MHC-II prevents recognition by CD4+ T-cells
The current, overall experience with nivolumab and pembrolizumab was summarized:
- >500 patients treated in phase IB and II studies
- Response rate is 65–70%, clinical benefit >90%
- CR rate 22% by investigator
- Median DoR unclear but >1 year
- Major side effects “itis”, endocrine or inflammatory
- Nivolumab approved in US in May for ASCT and BV failures
- Pembrolizumab likely to be approved in the next few months for refractory HL or relapse HL with >3 prior treatments
Dr Moskowitz then posed the question “should all transplant-eligible patients undergo HDT/ASCT with the availability of CPI?”
It was then asked if we have forgotten about allogeneic stem cell transplantation in this new era of CPI. It was noted that CPI have not cured any patients with HL yet. There are issues with how allogeneic transplantation and CPIs work together in current and future practice:
- 3-year PFS with allogeneic transplant varies in 2016 but ranges from 30–50%
- No patient in the US will be BV naïve if an allogeneic transplant is required
- Should an allogeneic transplant be offered to any patient that has not receive a CPI?
- Should an allogeneic transplant only be considered in patients that have disease progression on CPI?
- Should CPI be a bridge to allo in all cases?
- Should only patients that have <CR be referred for an allo?
- Should only patients with a CR be referred for an allo?
Dr Moskowitz then shared results by Reid W. Merryman et al. who investigated the safety and efficacy of allogeneic hematopoietic stem cell transplant following PD-1 blockade in relapsed/refractory lymphoma.
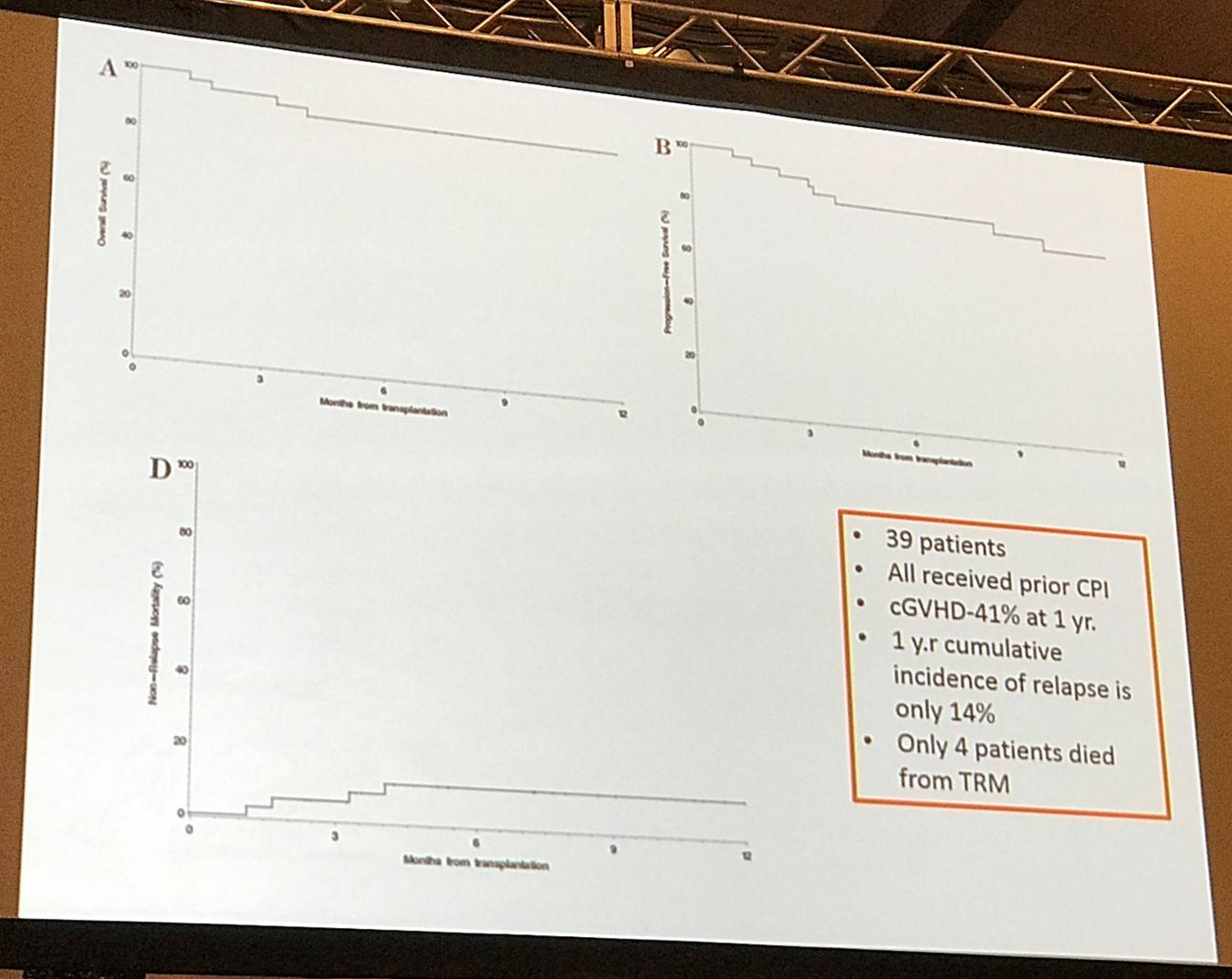
Finally, Dr Moskowitz shared his current strategy, which is subject to change, for patients who fail ASCT:

Abstract
The majority of patients with Hodgkin lymphoma are cured with frontline therapy; however, 10% to 15% with early-stage disease and 20% to 30% with advanced stage require second-line therapy that includes a potentially curative transplant, of which an additional 50% to 55% are cured. Those with multiply relapsed disease traditionally would receive novel agents on a clinical trial or combination chemotherapy as a potential bridge to an allogeneic stem cell transplant. This treatment paradigm has changed with the availability of brentuximab vedotin, an antibody drug conjugate used pre- and post-ASCT, as well as for palliation. With the availability of the checkpoint inhibitors, nivolumab and pembrolizumab, there will be another shift in treatment, with these agents being used for palliation and potentially replacing allogeneic stem cell transplantation in certain patient populations. Finally, up-front management is also changing and this will have an impact on how patients in the relapsed and refractory setting will be treated.
- Moskowitz C.H. Novel agents and strategies in transplant-eligible patients with relapsed and refractory Hodgkin Lymphoma. 2016 December 3; Educational Session Hodgkin Lymphoma: Treatment Today and the Challenge for Tomorrow. ASH 58th Annual Meeting and Exposition, San Diego, CA.
- Merryman R.W. et al. Safety and Efficacy of Allogeneic Hematopoietic Stem Cell Transplant Following PD-1 Blockade in Relapsed/Refractory Lymphoma. Blood 2015; 126:2018.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








