All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Incyte, and Lilly. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ASH 2016 | Brentuximab vedotin plus nivolumab is active and well tolerated in R/R cHL patients
On 3–6 December 2016, the Annual Meeting & Exposition of the American Society of Hematology (ASH) took place in San Diego, CA.
On Monday 5th December, an oral abstract session was held between 4:30pm and 6:00pm in the “Hodgkin Lymphoma and T/NK Cell Lymphoma – Clinical Studies Program: Oral and Poster Abstracts” category. This session was moderated by Stephen Ansell, MD, PhD, of the Mayo Clinic and Anas Younes, MD, of the University of Texas M.D. Anderson Cancer Center.
Abstract #1105 was presented during this session, titled “Preliminary Results from a Phase 1/2 Study of Brentuximab Vedotin in Combination with Nivolumab in Patients with Relapsed or Refractory Hodgkin Lymphoma” by Alex F. Herrera, MD, of the Department of Hematology/HCT, City of Hope National Medical Center, Duarte, CA, and colleagues.
The data presented in this abstract concerns a phase 1/2 (NCT02572167) evaluating the antitumor activity and safety of brentuximab vedotin plus nivolumab as first salvage therapy after standard frontline chemotherapy for Relapsed/Refractory (R/R) classical Hodgkin Lymphoma (cHL). Patients were administered with 1.8mg/kg brentuximab vedotin on Cycle 1 Day 1, and 3mg/kg nivolumab on Cycle 1 Day 8. Brentuximab vedotin and nivolumab were administered on Day 1 at the same doses on Cycles 2 through 4. Forty-two patients (52% female) have been enrolled, with a median age of 37 years. Twelve patients remain on treatment, 28 completed treatment and 9 initiated ASCT. Discontinuation prior to end of treatment occurred in 2 patients, due to investigator and patient decision (1 each).
- Pre-ASCT AEs occurring in ≥10% patients were mainly grade 1–2 and 1 grade 3 uticaria event
- Grade 1 peripheral sensory neuropathy took place in 3 patients (7%)
- After Cycle 1 BV, 1 patients experience treatment-related SAEs: grade 3 dehydration, grade 2 hypercalcemia and malaise, and grade 1 asthenia and nausea

- Infusion-Related Reactions (IRRs) were reported in 38% patients, most frequently reported symptoms were flushing, nausea (14% each); chest discomfort, dyspnea, uticaria (12% each); cough and pruritus (10% each)

- It was found that brentuximab vedotin plus nivolumab did not affect stem cell mobilization or engraftment

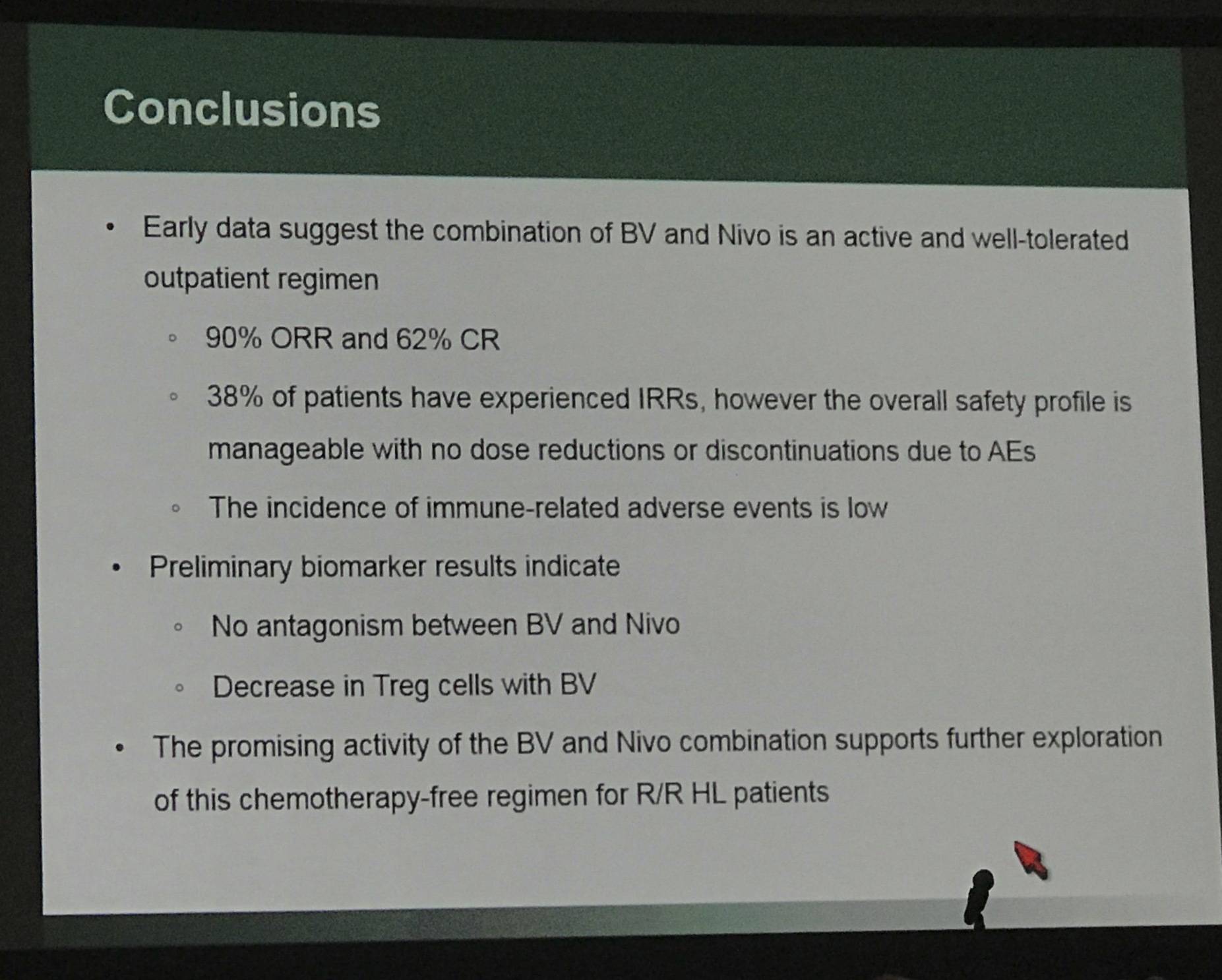
- ORR = 26/29 (90%; 95% CI: 72.6–97.8); CR = 18/29 (62%; 95% CI: 42.3–79.3)

- Peripheral blood immunophenotyping:


The results of this early data indicates that brentuximab vedotin combined with nivolumab is active and well tolerated as salvage therapy in R/R cHL patients.
Abstract:
Background: Brentuximab vedotin (BV) is an antibody-drug conjugate directed against CD30, a receptor that engages the CD30 ligand expressed on immune cells in the tumor microenvironment, promoting tumor cell growth (Montanari 2014, Hansen 2014). Through disruption of the microtubule network, BV induces apoptotic cell death, and may initiate an antitumor immune response (i.e., immunogenic cell death) through the induction of endoplasmic reticulum (ER) stress (Gardai 2015). Nivolumab is a fully human monoclonal PD-1 blocking antibody that prevents tumor immune evasion. Both agents have independent high single-agent response rates in patients (pts) with relapsed or refractory (R/R) Hodgkin lymphoma (HL), and when used in combination, may exhibit mechanistic synergy. Given the demonstrated efficacy of both BV and nivolumab, together these agents could yield improved CR rates prior to ASCT in pts with R/R HL, and potentially better long-term outcomes.
Methods: A phase 1/2 study is ongoing to evaluate the safety and antitumor activity of BV administered in combination with nivolumab as first salvage therapy in pts with R/R classical HL after standard frontline chemotherapy (NCT02572167). Adult pts are treated in 21-day cycles for up to 4 cycles (12 weeks). Pts receive 1.8 mg/kg BV on Cycle 1 Day 1, and 3 mg/kg nivolumab on Cycle 1 Day 8. For cycles 2 through 4, BV and nivolumab are administered on Day 1 of each cycle at the same doses. After completion of the Cycle 4 response assessment, pts are eligible to undergo ASCT. Investigator assessment of lymphoma response and progression is per the Lugano Classification Revised Staging System for malignant lymphoma (Cheson 2014).
Results: Twenty-five pts (60% female) with a median age of 32 years (range, 18-69) have been enrolled to date. Sixty percent of pts have relapsed disease, 36% have primary refractory disease (failure to achieve CR with frontline therapy, or relapse within 3 months of completing frontline therapy), and 1 pt (4%) has unknown status. At the time of enrollment, 32% of pts presented with extranodal disease and 16% with bulky disease.
At the time of the data extract, 23 pts had received treatment. An increased incidence of infusion-related reactions (IRRs) was observed at the start of combination treatment in Cycle 2 during the BV infusion leading to 1 dose delay. Premedication with corticosteroids (hydrocortisone 100 mg or equivalent) and antihistamines at Cycles 2-4 was instituted through a protocol amendment.
Six pts have completed combination treatment, and all have achieved an objective response (ORR, 100%), with 3 of 6 achieving a complete metabolic response (CmR, 50%). All 6 pts have proceeded directly to ASCT. Median number of CD34+ cells collected was 12.9 x106 cells/kg (range, 5-26) in a mean number of 1.7 apheresis sessions (range, 1-2).
Adverse events (AEs) for all pts are summarized prior to ASCT. Eighteen of the 23 treated pts (78%) experienced adverse events (AEs). Fifteen pts (65%) had AEs ≤ Grade 2 in severity and 3 pts (13%) experienced Grade 3 AEs. No pts experienced Grade 4 AEs pre-ASCT. Fatigue was the most common AE occurring in 35% of pts, followed by nausea (26%), rash (22%), dyspnea, myalgia, and pruritus (17% each). One pt experienced a treatment-related serious adverse event (SAE) of dehydration, hypercalcemia, and acute kidney injury. Immune-related adverse events (IrAEs) were experienced by 3 pts; 2 pts who experienced Grade 1 rash treated with topical steroids, and 1 pt who experienced Grade 1 hypothyroidism. No pts have discontinued treatment prematurely.
In addition to Reed-Sternberg cells, CD30 is expressed on activated T cells. Preliminary biomarker data indicate a BV-induced decrease in the percentage of CD4+ T regulatory (Treg) cells at C1D8, with no decrease in proliferating CD8+ T cells, and no significant decrease observed in the percentage of CD4+ Th1 cells compared to baseline for 5 of 6 pts (83%). Nivolumab induced a robust expansion of T cells at C1D15.
Conclusions: Early data suggest the combination of BV and nivolumab is an active and well-tolerated salvage therapy in pts with R/R HL. While an elevated incidence of IRRs has been observed, toxicities with this regimen appear to be tolerable overall. The preliminary antitumor activity suggests this combination may be a promising option for R/R HL pts. Updated results will be shared at the time of presentation.
References

