All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ASH 2016 | Phase II Study of Venetoclax Plus Rituximab or Randomized Ven Plus Bendamustine+Rituximab (BR) Versus BR in Patients with Relapsed/Refractory Follicular Lymphoma: Interim Data
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, and on December 5th, Pier Luigi Zinzani, MD, PhD, from the Institute of Hematology "L. e A. Seràgnoli", University of Bologna, Bologna, Italy, presented interim data from a Phase-II study into whether venetoclax + rituximab is more effective in treating R/R FL than either bendamustine + rituximab alone or venetoclax + bendamustine + rituximab.
Highlights:
- Three arm study:
- Arm A = Daily venetoclax 800mg for 1 year plus rituximab (cycles 1, 4, 6, 8, 10, 12)
- Arm B = Daily venetoclax 800mg for 1 year plus 6 cycles of bendamustine + rituximab
- Arm C = 6 cycles of bendamustine + rituximab only
- 164 R/R FL pts recruited – Arm A=53, Arm B=51, Arm C=51 (safety run-in=9)
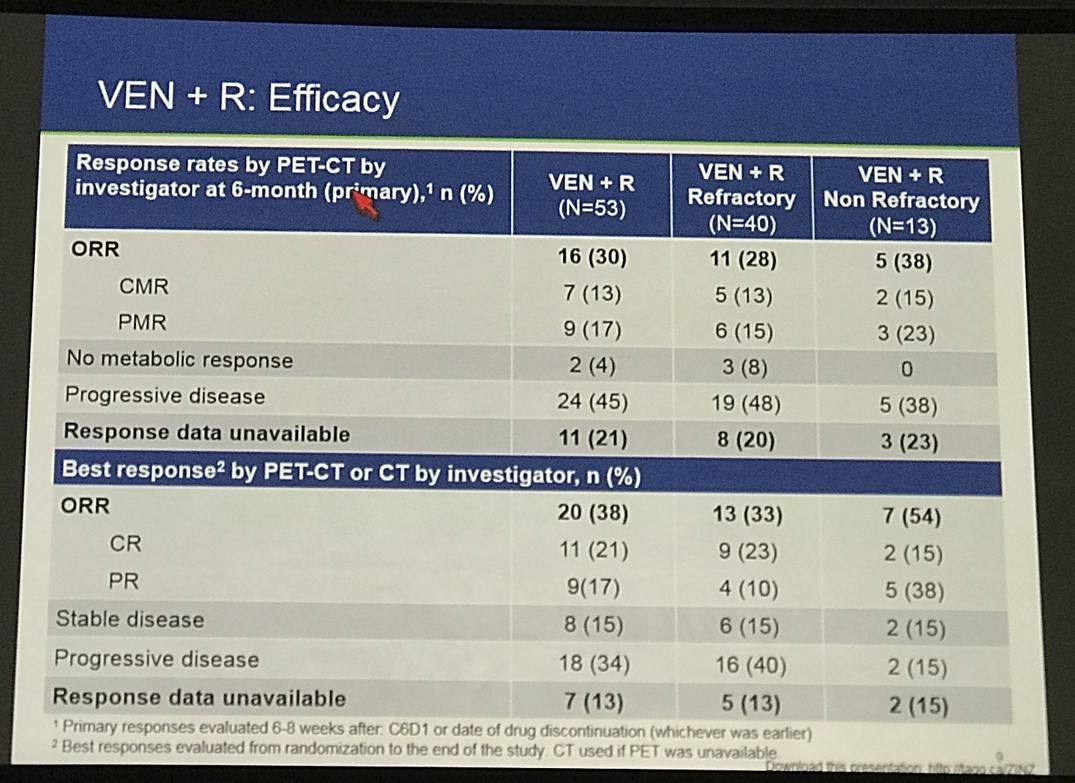
- Arm A Efficacy by PET-CT (V+R):
- ORR = 38%
- CR = 21%
- PR = 17%
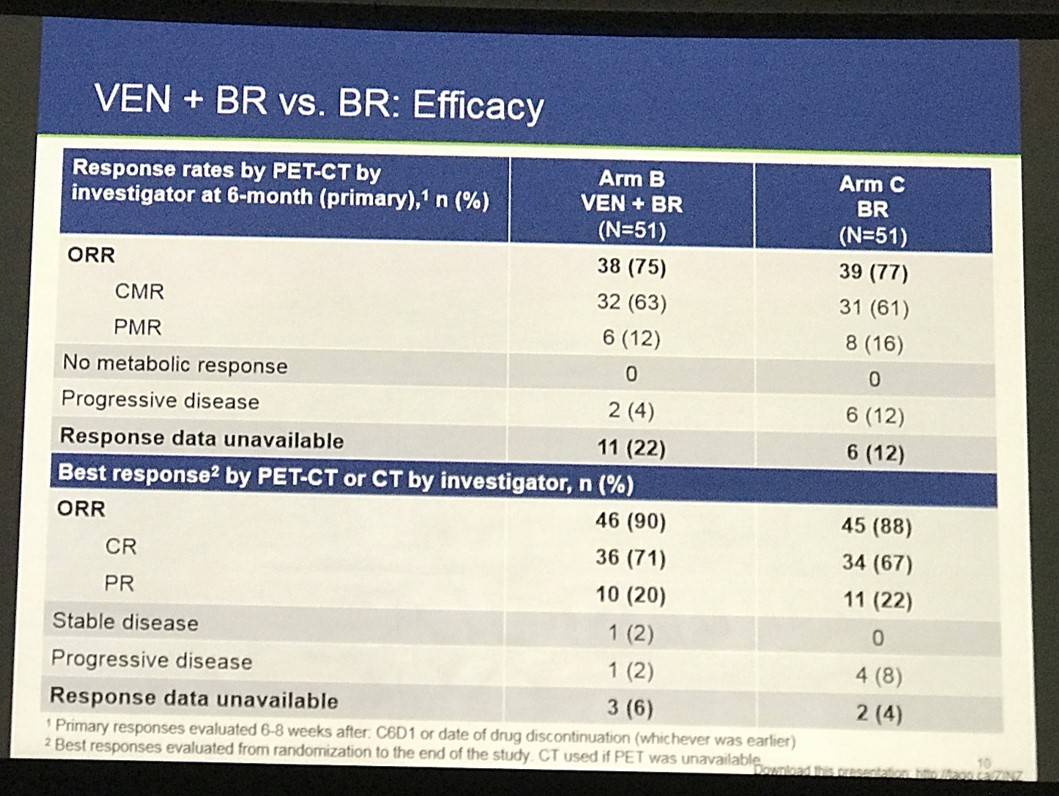
- Arm B Efficacy by PET-CT (V+B+R):
- ORR = 90%
- CR = 71%
- PR = 20%
- Arm C Efficacy by PET-CT (B+R)
- ORR = 88%
- CR = 67%
- PR = 22%
- PFS by PET or CT data was also presented, and PET-CT response at 6-months by BCL-2 status was also discussed
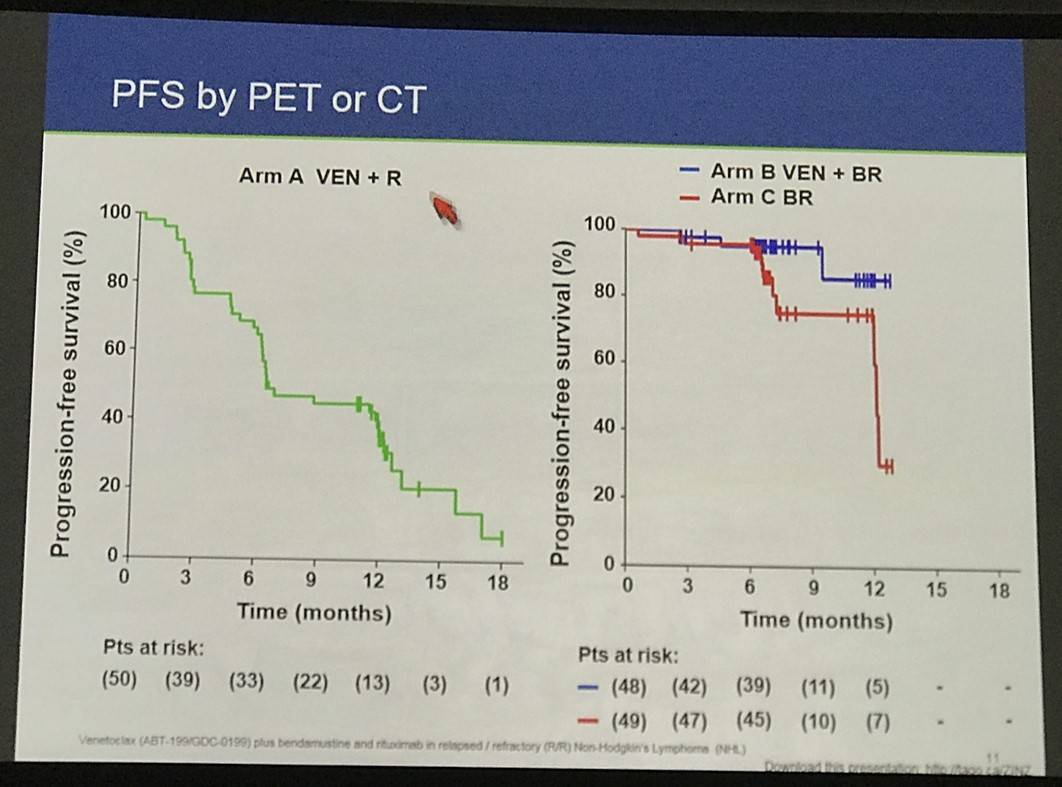
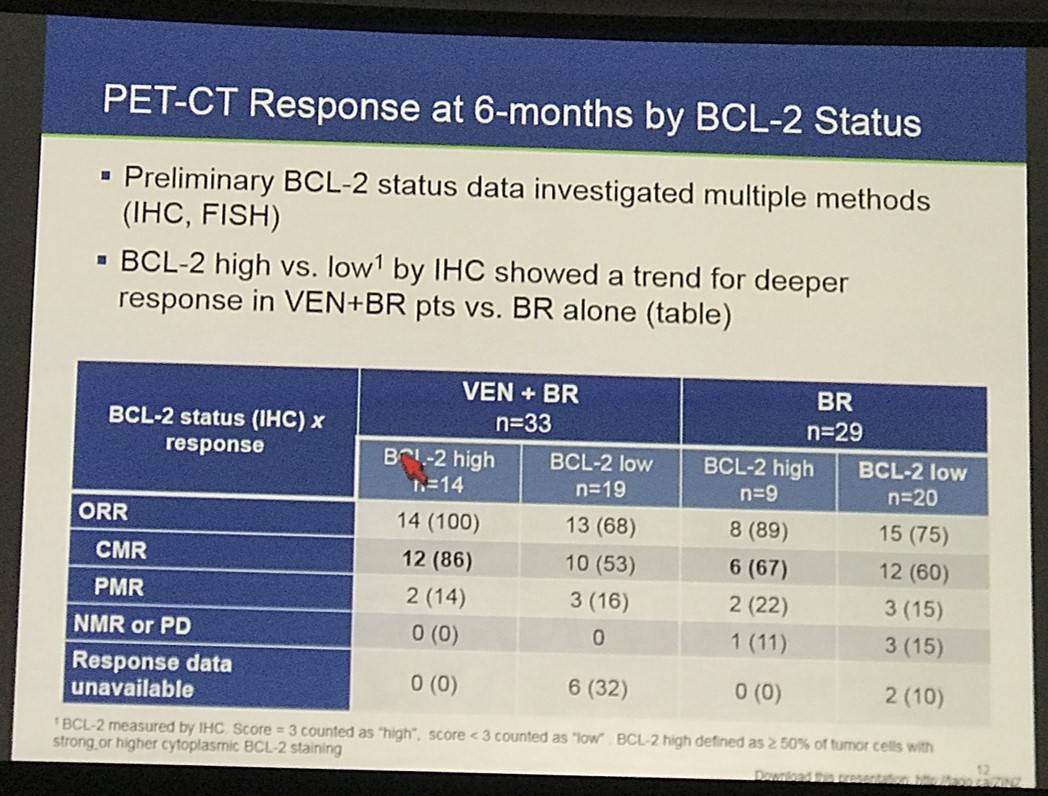
This abstract presentation was concluded by stating the combinations of venetoclax result in higher rates of hematologic toxicity, which can be manageable with proactive monitoring. Venetoxlax plus rituximab showed responses in R/R FL including CRs even in patients who were refractory to their last treatment. Preliminary efficacy data suggest an improvement in PFS when venetoclax is combined with BR compared to BR alone. Higher BCL-2 expression may be associated with a higher CR rate of venetoclax plus BR versus BR, consistent with the MoA of venetoclax. Lastly, longer follow-up is required to assess venetoclax plus BR as a potential new therapeutic option, and additional BCL-2 analyses are planned.
Abstract:
Introduction: Despite improved response and survival for patients (pts) with follicular lymphoma (FL) receiving rituximab (R) plus chemotherapy, new options are needed. FL is typically characterized by the presence of chromosomal translocation t(14;18), which results in overexpression of the anti-apoptotic protein BCL-2. BCL-2 may also contribute to resistance to chemoimmunotherapy such as bendamustine+R (BR), a regimen commonly used in FL. Venetoclax (VEN), a selective, potent oral BCL-2 inhibitor, is in development for the treatment of B-cell malignancies. Preclinical and early clinical data suggest VEN+R or VEN+BR may improve response over R or chemotherapy alone and is feasible. This open-label study will assess efficacy and toxicity of VEN+R as well as VEN+BR vs BR alone, in pts with relapsed/refractory (R/R) FL (NCT02187861).
Methods: R/R pts (age ≥18 yrs, confirmed Gr 1–3a FL, ECOG PS 0–2, adequate hematologic function) were assigned (at investigators’ discretion) to chemotherapy-free (chemo-free) or randomized to chemotherapy-containing (chemo) arms. Those in the chemo-free Arm (Arm A) received VEN 800mg daily for 1 yr + R (cycles [C] 1, 4, 6, 8, 10 and 12). After a safety run-in (9 pts at 600mg VEN daily + 6 cycles BR), those in the chemo Arm were randomized 1:1 to Arm B (VEN 800mg + 6 cycles BR) or C (BR only). The primary endpoint was complete response (CR) rate by PET-CT. Secondary endpoints include CR rate by CT and overall response rate. Response assessment was per Lugano non-Hodgkin lymphoma assessment (Cheson et al. 2014) 6–8 wks after D1 of C6 (or the cycle non-VEN agents were permanently discontinued: whichever came first).
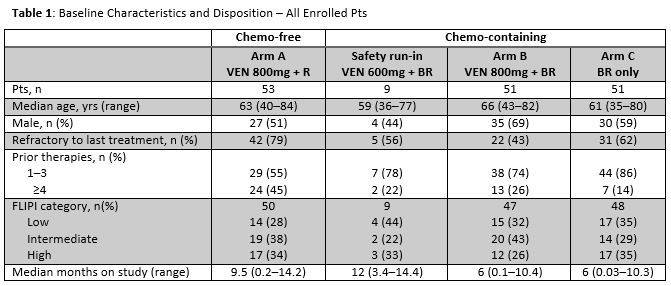
Results: One hundred sixty-four pts were enrolled as of May 13, 2016 (Table 1); 53 chemo-free (Arm A) and 111 chemo (9 run-in; 51 Arm B; 51 Arm C). 61% were refractory to prior treatment: 79% Arm A; 56% safety run-in; 43% Arm B; 62% Arm C.
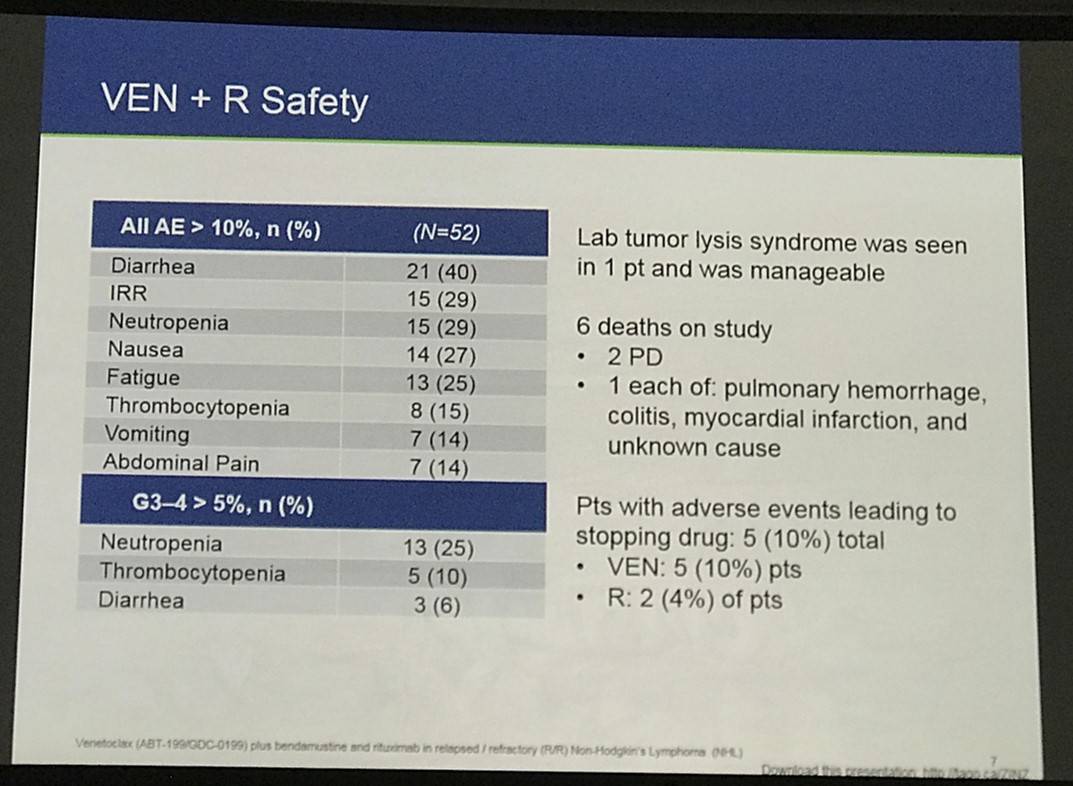
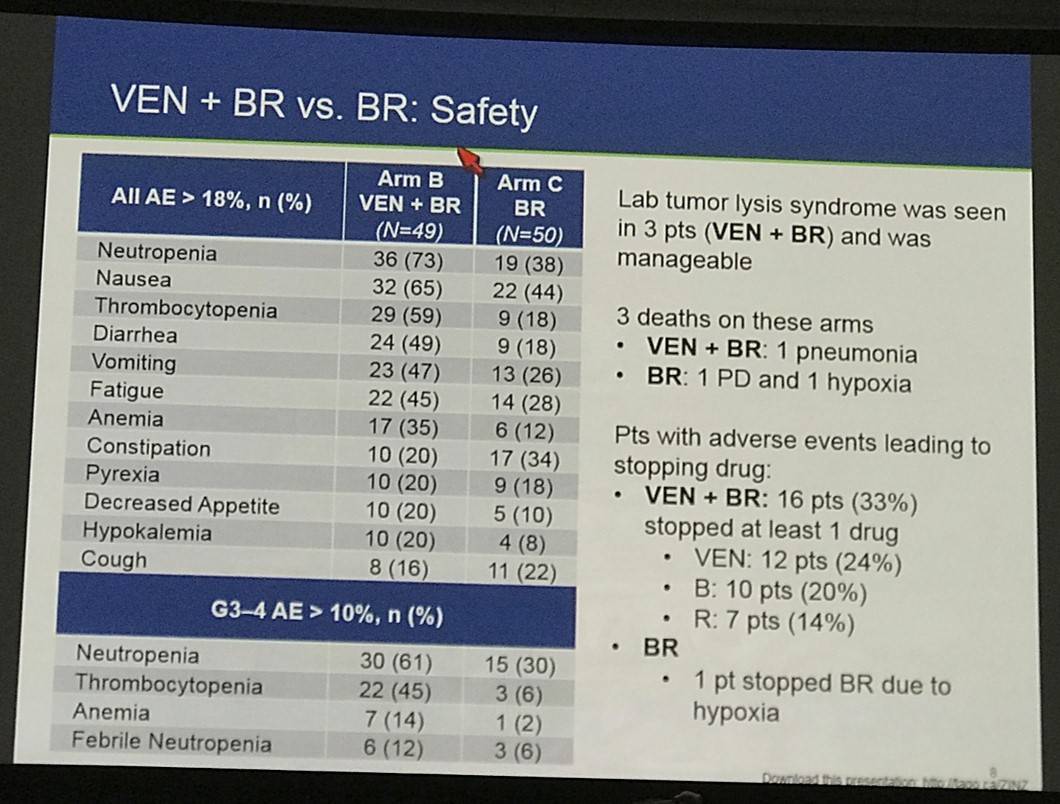
One hundred fifty-three of 160 safety evaluable pts (94%) experienced any AE (Table 2). Common AEs (all grades): diarrhea (37%), neutropenia (29%), nausea (25%), fatigue (25%), and IRR (25%). Common AEs for Arm B vs Arm C: nausea (61% vs 38%), neutropenia (51% vs 26%), diarrhea (41% vs 12%), thrombocytopenia (39% vs 20%) and fatigue (37% vs 24%). Neutropenia and thrombocytopenia were the most common Gr 3–4 AEs among all groups. 40 pts had serious AE (SAEs): 14 (27%) Arm A; 4 (44%) run-in; 15 (31%) Arm B; 7 (14%) Arm C. SAEs in >1 pt in any arm: febrile neutropenia (2 run-in; 5 Arm B; 1 Arm C), pneumonia (2 Arm A; 1 Arm B), and LDH increase (2 Arm A). Laboratory tumor lysis syndrome (TLS) events occurred in 2 pts (1 Arm A; 1 Arm B): both manageable and resolved with no clinical complications.
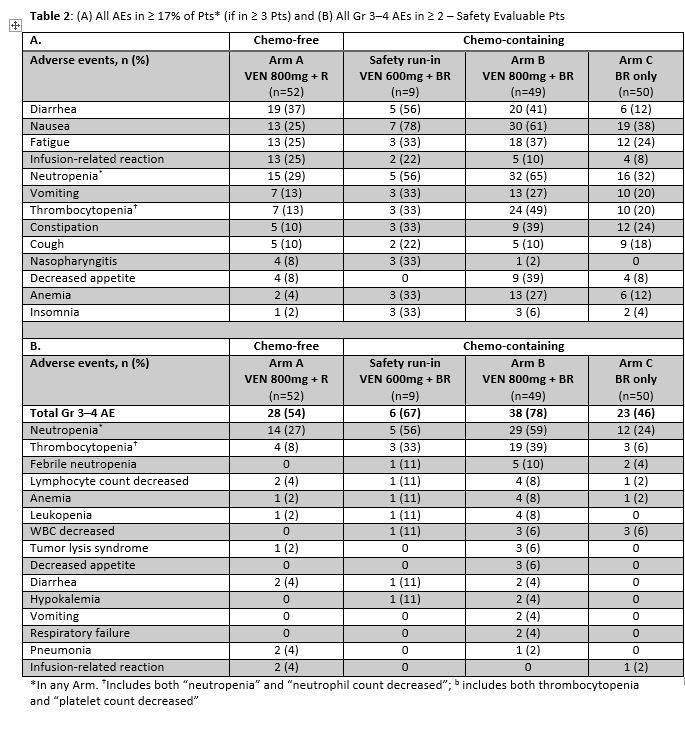
Five deaths occurred on study: 3 Arm A (colitis, pulmonary hemorrhage, PD), 1 Arm B (pneumonia), 1 Arm C (PD). Pts with AEs leading to dose interruptions or modification: 28 (54%) Arm A; 7 (78%) run-in; 37 (76%) Arm B; 12 (24%) Arm C. Pts with early drug discontinuations of VEN: 29 (56%) Arm A; 5 (56%) run-in; 11 (22%) Arm B. Early R discontinuations: 28 (54%) Arm A; 3 (33%) run-in; 7 (14%) Arm B; 5 (10%) Arm C. Early B discontinuations: 3 (33%) run-in; 7 (14%) Arm B; 4 (8%) Arm C.
Efficacy by PET+CT for safety evaluable patients who reached an assessment event is shown in Table 3. Arm A had 17 (33%) responders with 14% CR. Arm A was predominantly refractory to last treatment (79%): among non-refractory pts (21%), ORR was 64% with 27% CR. There was 68% ORR in Arm B with 50% CR compared with 64% ORR with 41% CR in Arm C. 55 (51%) of chemo pts have yet to reach a response-relevant event. Correlation with expression of BCL-2 family members is pending.
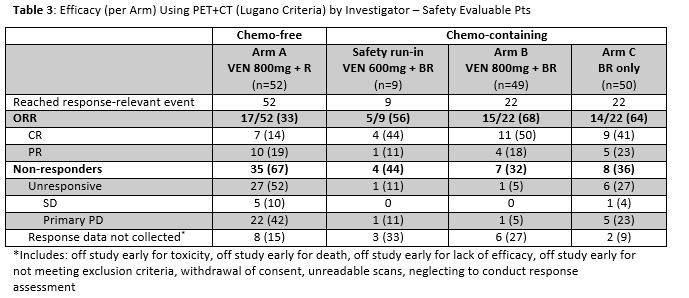
Conclusion: Early results from the first study evaluating VEN+R and VEN+BR in pts with FL show that daily VEN 800mg has an acceptable benefit/risk ratio. As expected with the known VEN safety profile, both VEN+R and VEN+BR are associated with hematologic and GI toxicity, which appears manageable. Laboratory TLS was rare (<2%) and manageable with no clinical sequelae. Objective response with VEN+R is 33% even in a highly refractory pt population and 64% among non-refractory pts, providing a potential chemotherapy-free option for both groups. While early data shows VEN+BR is associated with more toxicity and some early treatment discontinuations compared with BR alone, data suggest it may be a tradeoff for increased CR and decreased PD. Future data will help determine whether continued VEN treatment beyond initial chemotherapy deepens response.
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

