All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Minimal Residual Disease in Patients with Follicular Lymphoma Treated with Obinutuzumab or Rituximab as First-Line Induction Immunochemotherapy and Maintenance in the Phase 3 GALLIUM Study
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, and on December 3rd, Christiane Pott from University Hospital Schleswig-Holstein, Kiel, Germany, presented the results of the Phase-III GALLIUM study, focusing on Minimal Residual Disease (MRD) results at mid-induction and end-of-induction time points. The GALLIUM study is a randomized, global, Phase-III trial comparing induction rituximab (R) or obinutuzumab (G) in combination with chemotherapy (either bendamustine, CVP or CHOP) in FL patients.
Highlights:
- 1138pts with FL with consent for MRD analysis
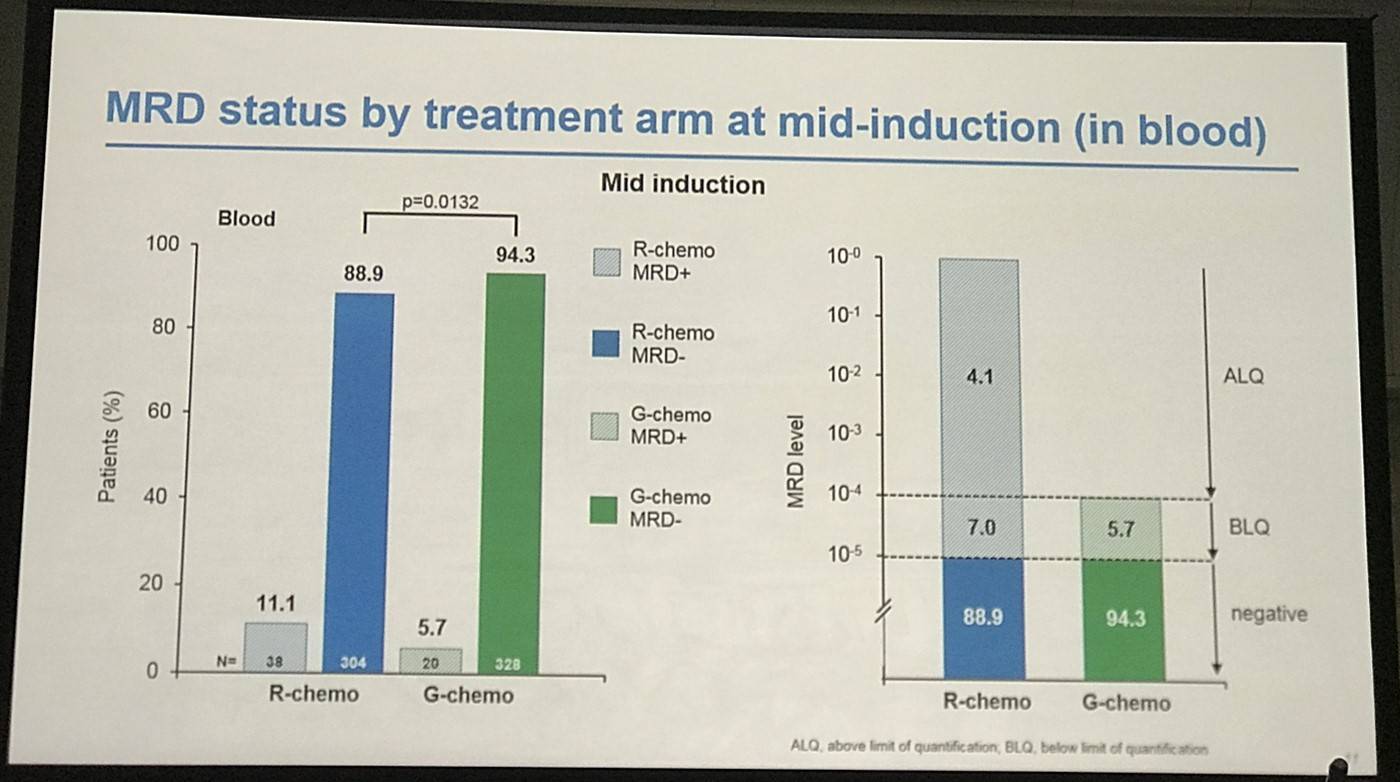
- Mid-Induction:
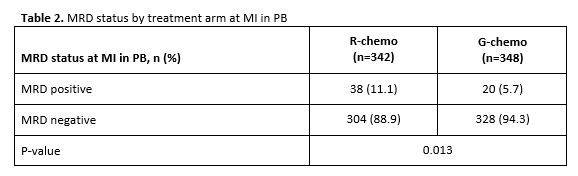
- MRD clearance: R-chemo=89%, G-chemo=94% (P=0.013)
- 63% pts still MRD-positive in R-chemo group (n=38) had unquantifiable MRD (below quantifiable levels) compared with 100% G-chemo group (n=20)
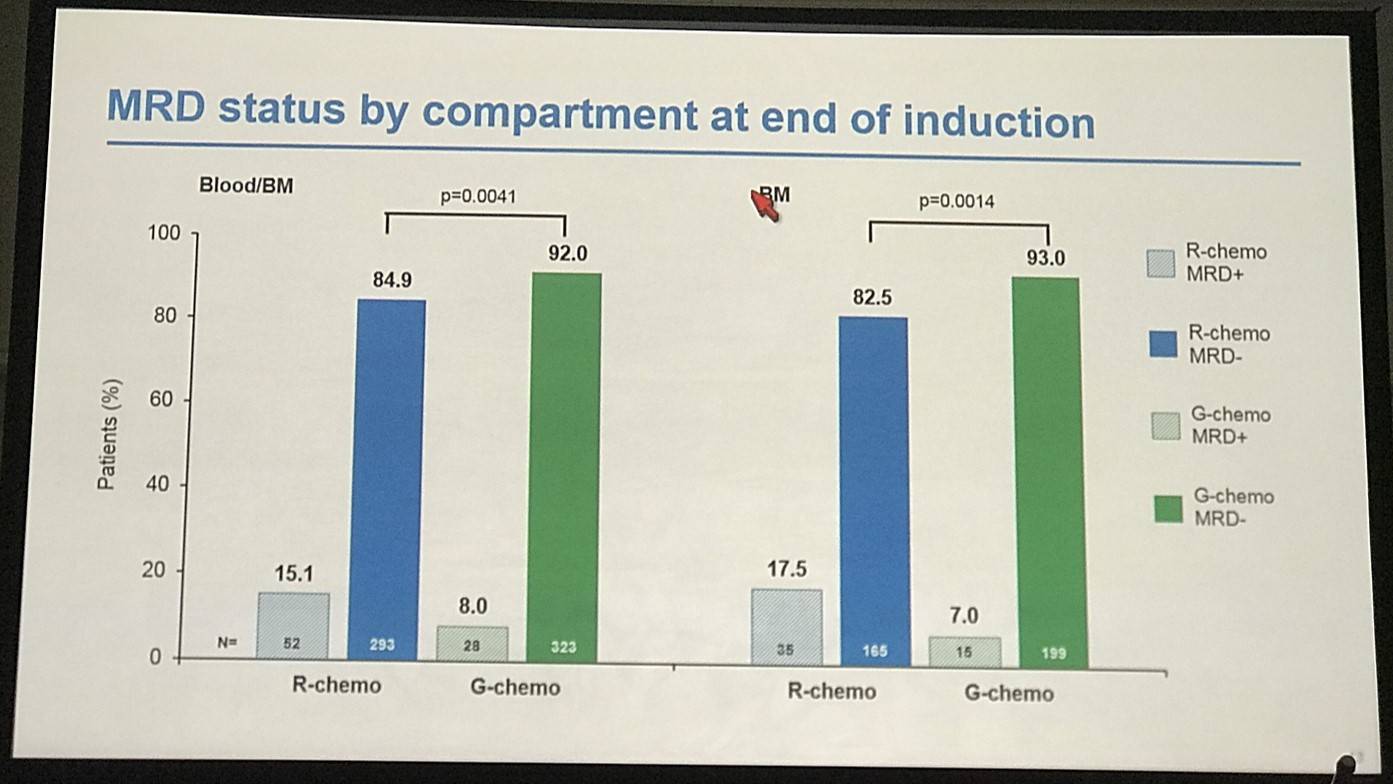
- End-of-Induction:
- MRD response R-chemo=85%, G-chemo=92% (P=0.0041)
- Residual tumor cell bone marrow clearance: R-chemo=83%, G-chemo =83% (P=0.0014)
- Residual tumor cell peripheral blood clearance not significantly different
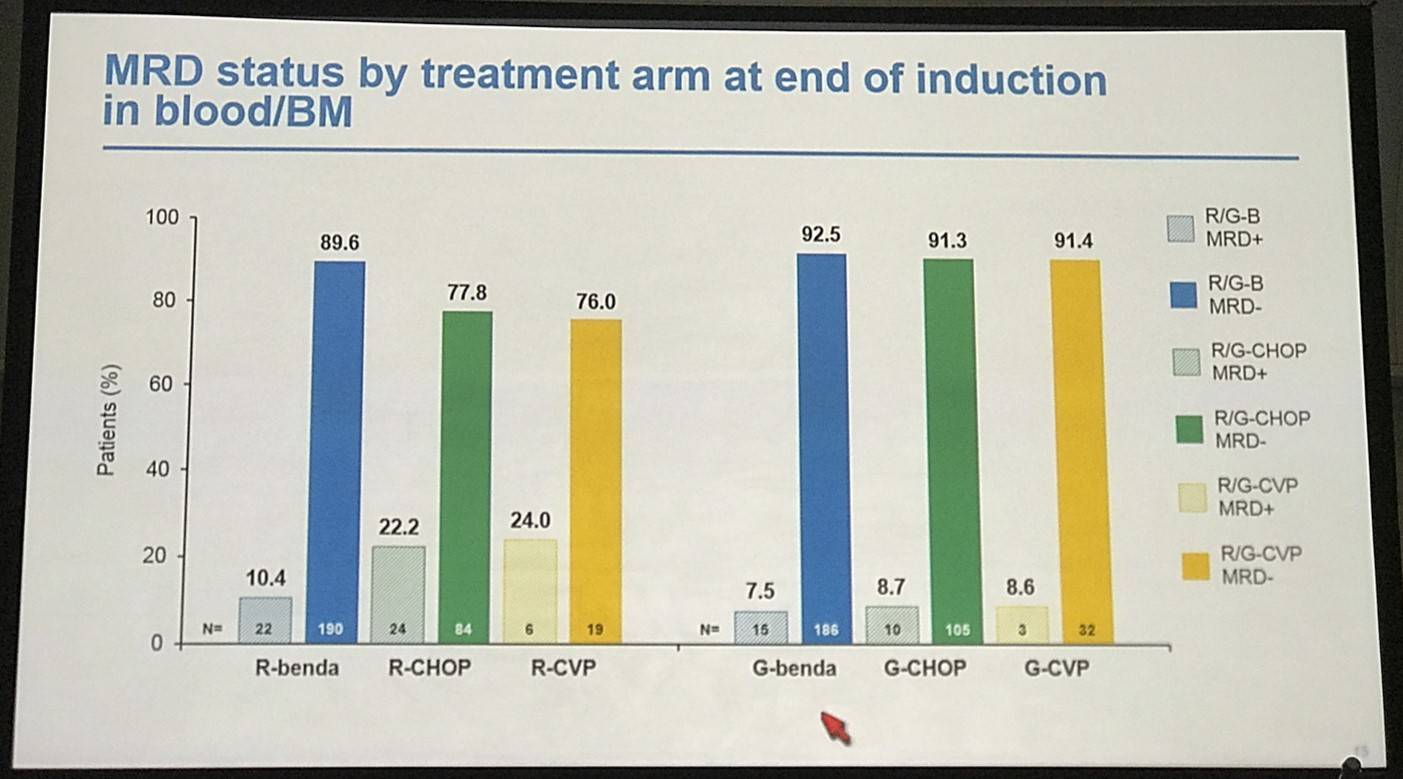
- R-chemo combination peripheral blood MRD response rates:
- R-CVP=79% (n=24)
- R-CHOP=93% (n=108)
- R-bendamustine=96% (n=209)
- G-chemo combination peripheral blood MRD response rates:
- R-CVP=94% (n=33)
- R-CHOP=96% (n=109)
- R-bendamustine=96% (n=194)
- Complete or partial response combined with peripheral blood or bone marrow MRD negative status at end-of-induction associated with longer PFS HR=0.35% (95% CI, P<0.0001)

Christiane Pott concluded by stating that obinutuzumab induction was more effective and faster than rituximab at clearing tumor cells from both peripheral blood and bone marrow in combination with chemotherapy. Additionally, EOI MRD status could have prognostic value in FL patients following immunochemotherapy.
Abstract:
Background: Minimal residual disease (MRD) status reflects treatment efficacy and may predict prognosis after first-line therapy in patients (pts) with follicular lymphoma (FL). GALLIUM (NCT01332968) is an ongoing, randomized global Phase 3 study evaluating the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) vs rituximab (R) as induction (with chemotherapy [chemo]; bendamustine, CHOP or CVP) and maintenance (in responders) in previously untreated pts with indolent non-Hodgkin lymphoma. In GALLIUM, the primary endpoint of investigator-assessed PFS in pts with FL was significantly improved with G- vs R-based treatment. We report the results of MRD assessment at mid induction (MI) and end of induction (EOI) in FL pts in GALLIUM.
Methods: Diagnostic peripheral blood (PB) and bone marrow (BM) samples from FL pts were screened by consensus PCR to detect a t(14;18) translocation and/or clonal Ig variable domain rearrangement suitable for MRD assessment. In pts with a detectable clonal marker, allele- or translocation-specific real-time quantitative (RQ)-PCR assays were designed with a sensitivity ≤10-4. RQ-PCR data were evaluated by European Study Group criteria for MRD detection (van der Velden, Leukemia 2007). MRD status was assessed at MI in PB, and at EOI in PB and BM, and defined as negative (MRD response) if RQ-PCR and subsequent nested PCR were negative in all samples analyzed at the respective time point.
Results: Of the 1202 FL pts enrolled in GALLIUM, 1138 provided consent for MRD analyses. Baseline PB or BM samples were available for 1101 pts; a clonal marker was detected in 968 (88%) of these pts and 815 (74%) had an RQ-PCR assay fulfilling sensitivity criteria. Pts with a detectable clonal marker had baseline characteristics comparable to pts without a marker, with the exception of higher-stage disease (61% vs 34% Ann Arbor stage IV), reflecting increased BM involvement. Among 696 pts with an available PB or BM sample at EOI, MRD response was significantly higher in the G-chemo arm than the R-chemo arm (92% vs 85%; p=0.0041; Table 1). BM clearance of residual tumor cells at EOI was higher in the G-chemo vs R-chemo arm (93% [199/214] vs 83% [165/200]; p=0.0014), whereas PB clearance at EOI was similarly high in both arms (96% [323/336] vs 94% [320/341]; p=0.22). MRD clearance occurred early during treatment: at MI, 94% of pts in the G-chemo arm achieved MRD-negative status in PB compared with 89% in the R-chemo arm (p=0.013; Table 2). The anti-lymphoma activity of G-chemo induction was confirmed by analyzing quantitative MRD data in PB at MI: all 20 (100%) pts who remained MRD-positive at MI in the G-chemo arm had low-level MRD (below the limit of quantification) compared with 24/38 (63%) pts in the R-chemo arm. The chemo backbone in the R-chemo arm affected MRD status in PB at EOI giving an MRD response rate of 96% (201/209) after R-bendamustine (B), 93% (100/108) after R-CHOP, and 79% (19/24) after R-CVP. No such effect was seen in the G-chemo arm, where MRD response rates in PB at EOI were high and similar with all three chemo regimens: 96% (187/194), 96% (105/109), and 94% (31/33), respectively. Similarly, in BM, chemo had a large influence on MRD-negative status at EOI in the R-chemo arm (87% [100/115] after R-B, 74% [55/74] after R-CHOP), but negligible impact in the G-chemo arm (93% [109/117] after G-B, 93% [76/82] after G-CHOP). Achievement of MRD negativity at EOI in PB/BM for pts with CR/PR at EOI was associated with longer subsequent PFS, with a hazard ratio of 0.35 (95% CI, 0.22, 0.56; p<0.0001; Figure 1), and comparable effects in both treatment arms.
Conclusions: Data from this exploratory analysis support the potential prognostic value of MRD assessment after EOI in FL pts treated with immunochemotherapy. The higher proportion of pts achieving MRD-negative status at MI and EOI, and of pts with low-level MRD among MRD-positives at MI, in the G-chemo arm suggests that G-based induction induces rapid and more effective tumor cell clearance than R-containing therapy. G was more effective than R at enhancing the depth of response to induction therapy in PB and BM, potentially leading to a compensation of differential chemo activity. Future analyses of MRD kinetics during maintenance/follow-up will investigate the pattern and prognostic value of changes in MRD status before relapse, and further evaluate the impact of different chemo regimens.
Figure 1. PFS from date of EOI sample by MRD status in PB and/or BM
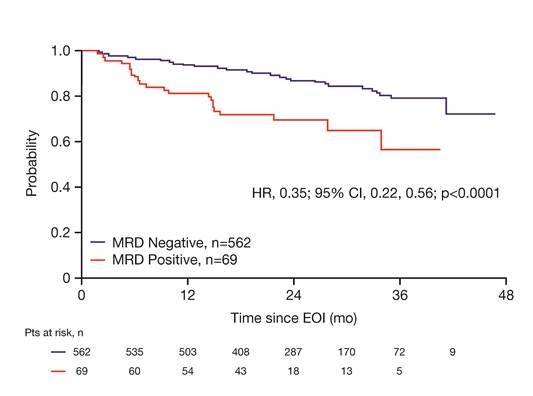
Table 1. MRD status by treatment arm at EOI in PB and/or BM
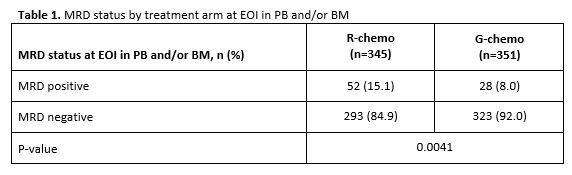
Table 2. MRD status by treatment arm at MI in PB
- Pott C. et al. Minimal Residual Disease in Patients with Follicular Lymphoma Treated with Obinutuzumab or Rituximab as First-Line Induction Immunochemotherapy and Maintenance in the Phase 3 GALLIUM Study. 2016 December 3; Oral Abstract #613: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








