All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Brentuximab-vedotin and bendamustine salvage therapy in patients with relapsed/refractory PTCL
Peripheral T-cell lymphoma (PTCL) is an aggressive form of lymphoma. Patients with relapsed/refractory (R/R) PTCL have a poor prognosis, and there remains a medical need for an efficacious salvage therapy. While bendamustine and brentuximab-vedotin (Bv) have demonstrated efficacy as single agents in patients with R/R PTCL, there is limited data on their combined efficacy.
Here, we summarize a retrospective study published by Aubrais et al.1 evaluating the safety and efficacy of bendamustine plus Bv (BBv) combination in patients with R/R PTCL.
Study design
Patients with R/R PTCL treated with BBv at 21 LYSA centers between January 2013 and October 2020 were included in this retrospective study. The study design is shown in Figure 1.
Figure 1. Study design*
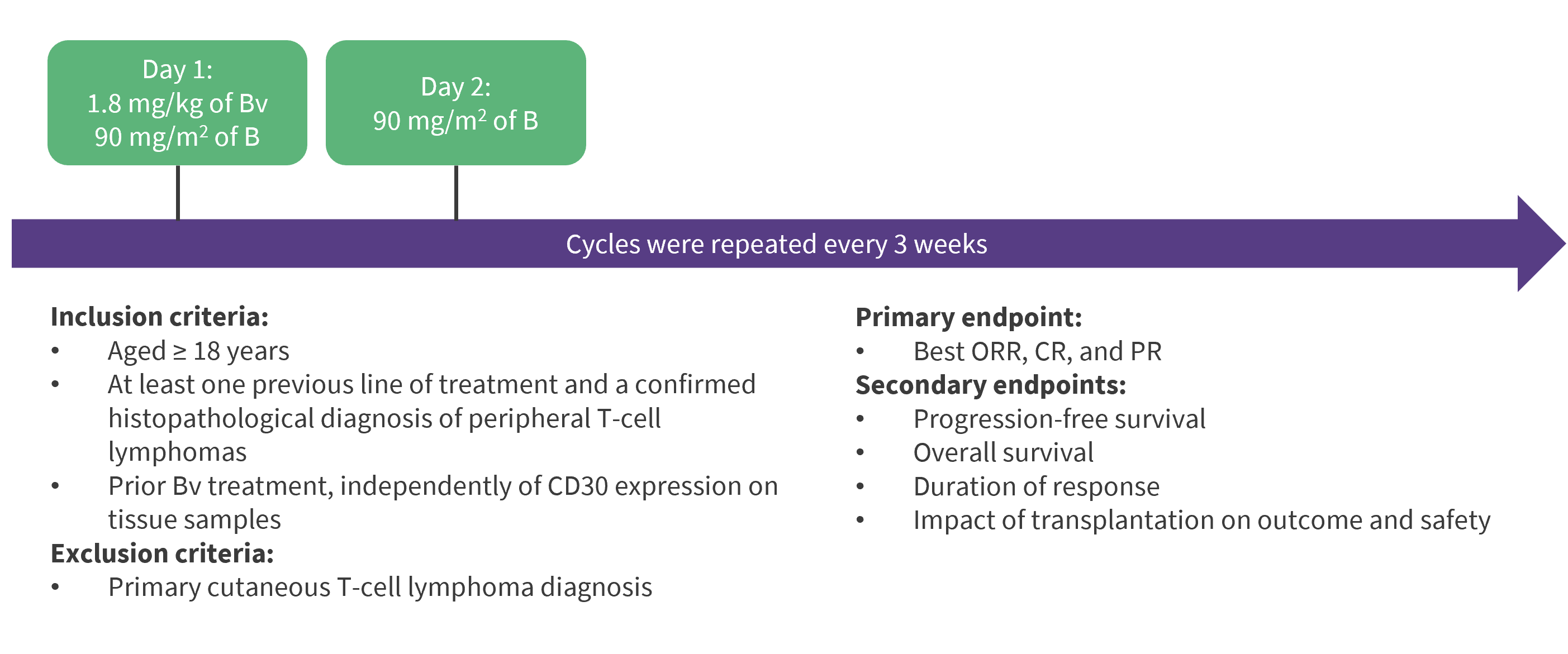
B, bendamustine; BBv, bendamustine and brentuximab-vedotin; Bv, brentuximab-vedotin; CR, complete response; ORR, overall response rate; PR, partial response; PTCL, peripheral T-cell lymphomas.
*Data from Aubrais, et al.1
Baseline characteristics
A total of 82 patients were included with a median age of 60 years (range, 25–85 years), and 85% of patients were aged ≤70 years. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
ALCL, ALK + anaplastic large-cell lymphoma; Bv, brentuximab-vedotin; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; EATL, enteropathy associated T-cell lymphoma; HDACi, histone deacetylase inhibitor; HSCT, hematopoietic stem cell transplantation; IPI, International Prognostic Index; TFH, T follicular helper; T/NK extranodal, extranodal natural killer/T-cell lymphoma; PTCL NOS, peripheral T-cell lymphoma not otherwise specified. |
|
|
Characteristic, % (unless otherwise stated) |
Number of patients (N = 82) |
|---|---|
|
Sex |
|
|
Male |
61 |
|
Female |
39 |
|
Lymphoma histology |
|
|
TFH |
51 |
|
PTCL NOS |
16 |
|
ALCL |
27 |
|
EATL |
4 |
|
T/NK extranodal |
1 |
|
Subcutaneous panniculitis |
1 |
|
CD30 status† |
|
|
Positive |
63 |
|
Negative |
26 |
|
Stage |
|
|
1–2 |
12 |
|
3–4 |
87 |
|
IPI |
|
|
0–2 |
49 |
|
3–5 |
37 |
|
Median number of previous regimens (range), n |
1 (1–6) |
|
Disease status at last regimen |
|
|
Refractory |
50 |
|
Early relapse (<1 year) |
35 |
|
Late relapse (≥1 year) |
15 |
|
Previous therapy |
|
|
CHOP-like regimen |
96 |
|
Cytarabine and/or platine-based |
35 |
|
Other polychimiotherapy |
16 |
|
New treatments |
|
|
HDACi |
5 |
|
Bv |
11 |
|
Lenalidomide |
2 |
|
HSCT |
30 |
Key findings1
Efficacy
Overall, 81 patients were evaluable for response (lost to follow-up, n = 1). The response to BBv is shown in Figure 2, and the median duration of response, progression-free survival (PFS), and overall survival (OS) are shown in Figure 3.
Figure 2. Response to BBv*
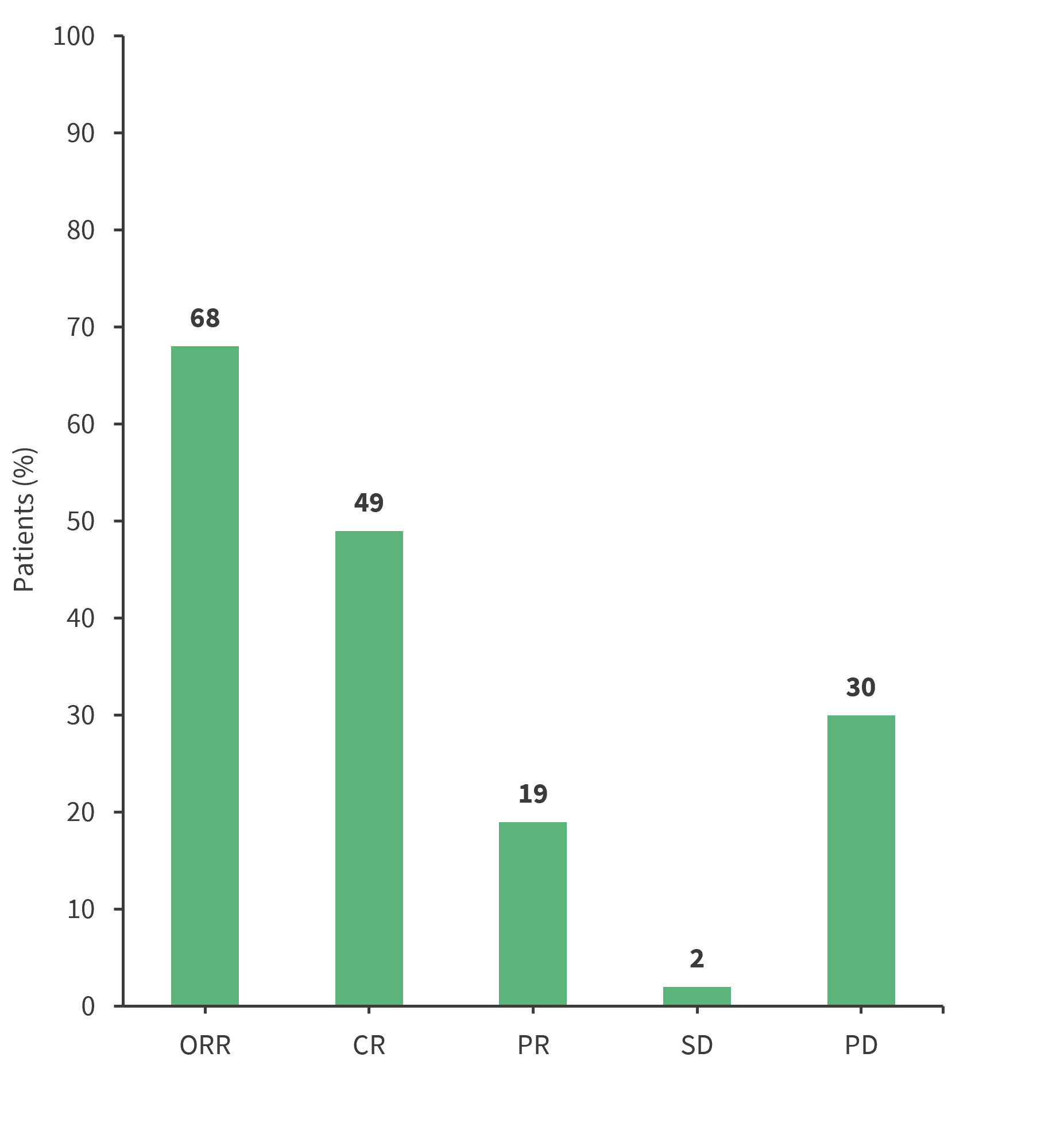
BBv, bendamustine plus brentuximab-vedotin; CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
*Data from Aubrais, et al.1
Figure 3. Median DoR, PFS, and OS*
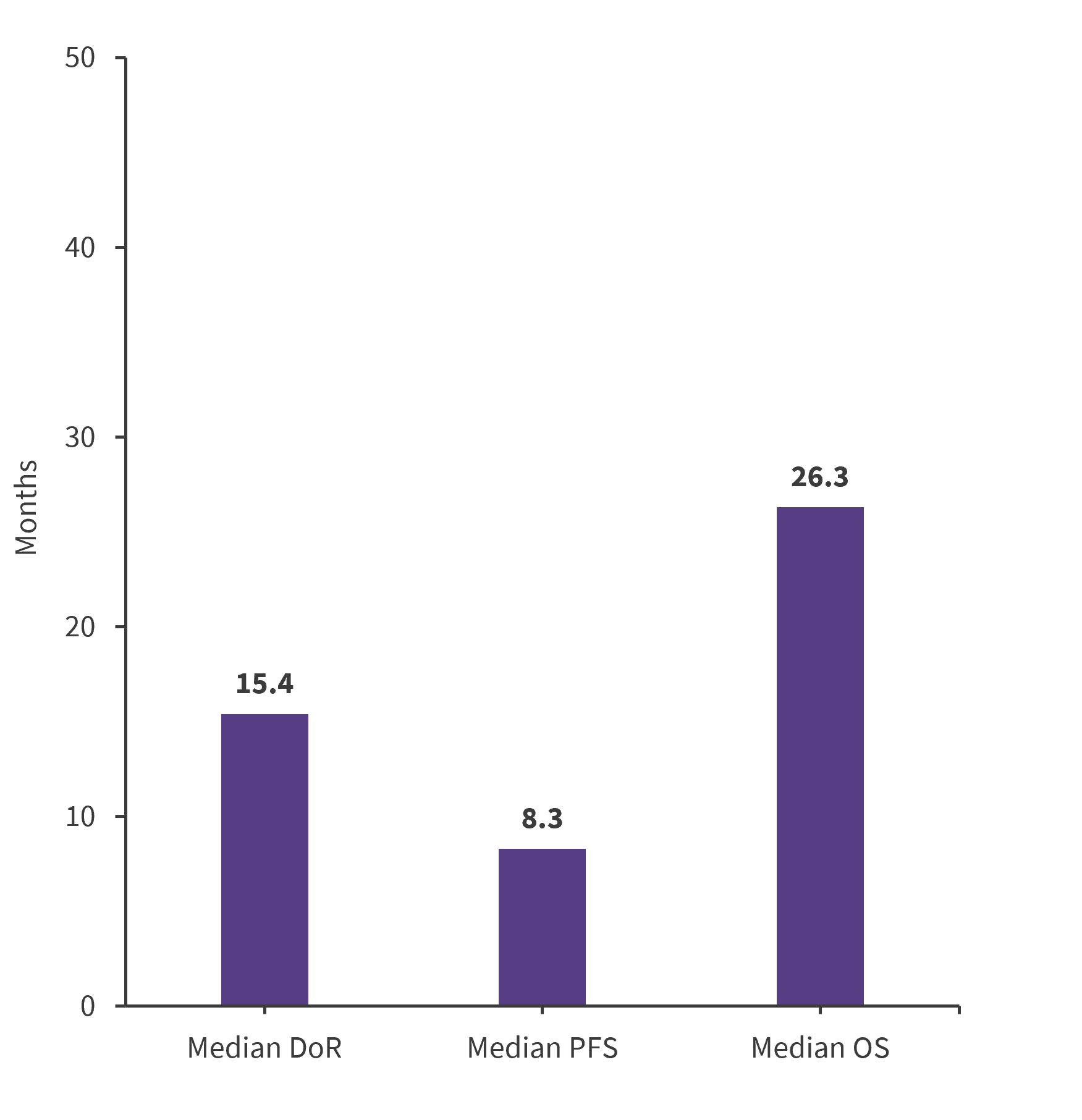
DoR, duration of response; OS, overall survival; PFS, progression-free survival.
*Data from Aubrais, et al.1
Predictive factors for response
Disease status after the last regimen (relapse vs refractory) and International Prognostic Index at relapse (0–2 vs 3–5) were associated with a better overall response rate (ORR) in the univariate analysis.
In the multivariate analysis, only disease status remained significantly associated with response; patients with relapsed disease had a better overall response rate compared with patients who had refractory disease (83% vs 53%; odds ratio, 3.70; 95% confidence interval [CI], 1.3–10.5; p = 0.014).
The median duration of response was significantly increased in patients who achieved complete response and underwent transplantation vs those who did not (p = 0.0055).
Predictive factors for survival
Hematopoietic stem cell transplantation, type of response, histological subtype, and International Prognostic Index at relapse were significantly associated with better PFS and OS in the univariate analysis.
In the multivariable analysis, response to treatment and transplantation had a significant impact on PFS and OS:
- Median PFS and OS (p < 0.0001) were significantly longer in patients who achieved a good response (complete response or partial response) vs those who did not.
- Median PFS (p = 0.0005) and OS (p = 0.0013) were also longer in patients who underwent allogeneic hematopoietic stem cell transplantation vs those who did not, regardless of their response status.
Safety
Grade 3 to 4 adverse events were reported in 59% of patients. The most frequent adverse events were hematologic, infectious, and neurologic toxicities, as shown in Figure 4. Doses had to be reduced in 33% of patients, mainly due to hematologic toxicities, neurotoxicity, rash, and gastrointestinal toxicity. Treatment had to be stopped early in 11% of patients due to hematologic toxicity and neurotoxicity.
Figure 4. Adverse events*
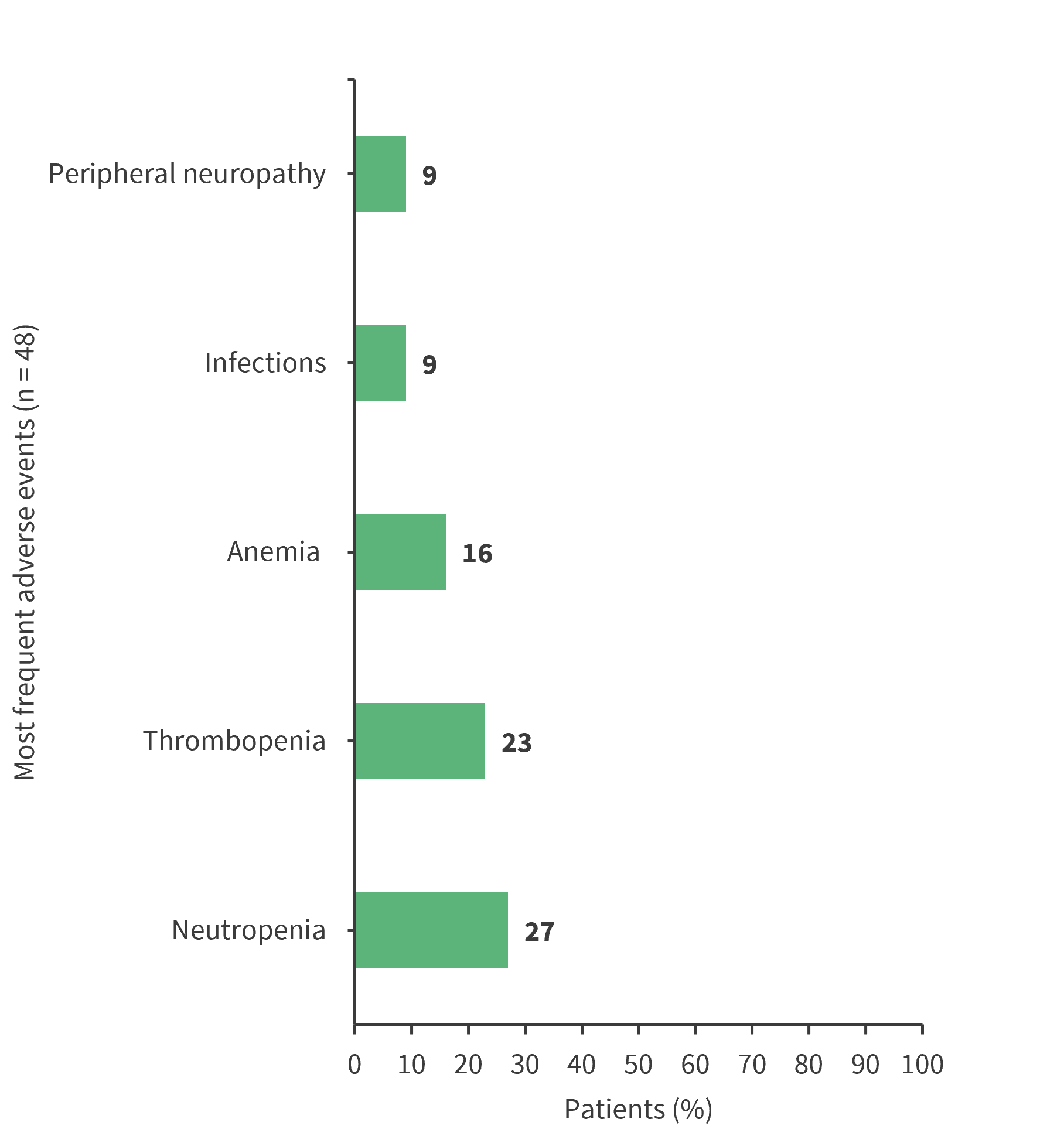
*Data from Aubrais, et al.1
Conclusion
This retrospective study demonstrated BBv as a promising salvage therapy, improving outcomes in patients with R/R PTCL. Further evaluation in prospective studies is needed to establish BBv as standard of care in this setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

