All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Brentuximab vedotin in combination with standard chemotherapy for pediatric patients with newly diagnosed, ALK+ ALCL
Anaplastic large-cell lymphoma (ALCL) is a type of non-Hodgkin lymphoma that accounts for 10–15% of all childhood lymphomas. It is characterized by pleomorphic tumor cells and strong expression of CD30. ALCL can be classified into two groups based on the expression of the ALCL kinase (ALK). ALK+ ALCL accounts for over 95% of pediatric ALCL. Although numerous treatment strategies have been investigated, approximately 30% of pediatric patients with ALCL relapse.1
Brentuximab vedotin (BV) consists of an anti-CD30 antibody conjugated to monomethyl auristatin E (MMAE); this antibody-drug conjugate has demonstrated excellent activity as a single agent in adults with ALCL and children with relapsed ALCL, though it has not been investigated in newly diagnosed pediatric patients. Therefore, the Children’s Oncology Group (COG) trial ANHL12P1 (NCT01979536) was initiated to investigate the toxicity and efficacy of BV or crizotinib in combination with standard chemotherapy (ALCL99 protocol) in children with newly diagnosed, non-localized, ALK+/CD30+ ALCL. Results of the BV arm were published by Eric Lowe and colleagues in the journal Blood and are summarized below.1
Study design and patient characteristics1
- This was a partially randomized, phase II trial to determine the toxicity and efficacy of the addition of BV or crizotinib to standard chemotherapy in pediatric patients (<22 years of age) with newly diagnosed, non-localized, ALK+/CD30+ ALCL.
- Sixty-eight patients were enrolled in the BV arm between November 8, 2013, and January 20, 2017, across 52 centers.
- The data cutoff was September 30, 2019.
- Forty-eight patients were randomized to the BV arm and 20 patients with body surface area (BSA) <0.9 m2 were nonrandomly assigned to the BV arm, due to difficulties in dosing crizotinib in these patients.
- The dosing schedule can be seen in Figure 1. All patients received a 5-day prophase followed by standard chemotherapy with the addition of BV.
Figure 1. Dosing schedule*
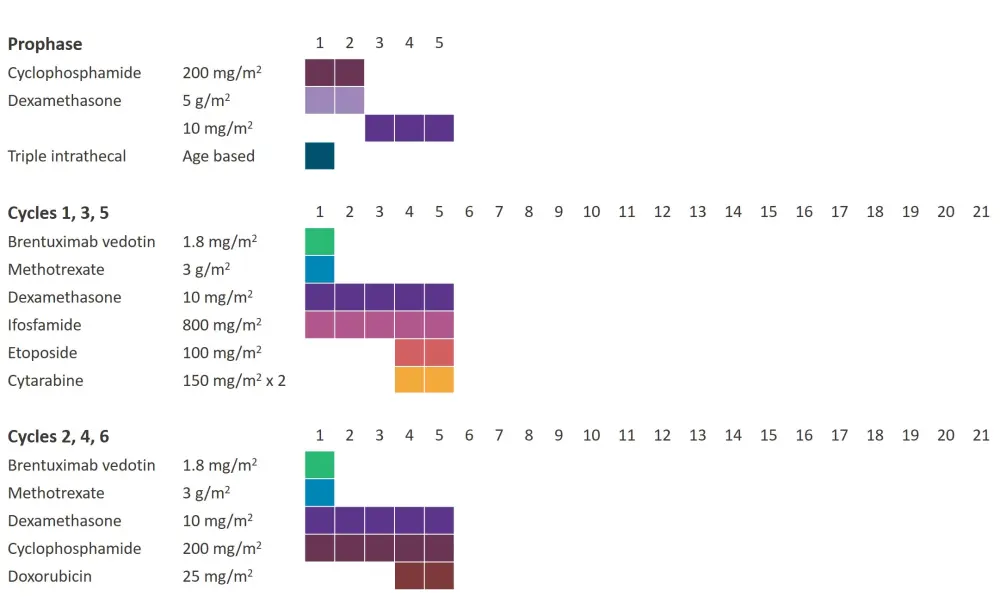
*Data from Lowe et al.1
- Baseline characteristics can be seen in Table 1.
- Median age at enrollment was 12 years.
- There were slightly more males than females (63.2% vs 36.8%).
- Extranodal sites of disease were common; 61.8% of patients had ≥1 extranodal site of disease.
Table 1. Baseline characteristics*
|
*Adapted from Lowe et al.1 |
|
|
Characteristic |
N = 68 |
|---|---|
|
Median age (range), years |
12 (2–21) |
|
Age group, % |
|
|
<6 years |
19.1 |
|
6–12 years |
36.8 |
|
≥13 years |
44.1 |
|
Male/female, % |
63.2/36.8 |
|
Stage, % |
|
|
II |
16.2 |
|
III |
70.6 |
|
IV |
13.2 |
|
Site of disease, % |
|
|
Lymph node |
95.6 |
|
Bone |
23.5 |
|
Bone marrow |
13.2 |
|
Liver |
1.5 |
|
Lung |
13.2 |
|
Skin |
4.4 |
|
Soft tissue |
29.4 |
|
Spleen |
10.3 |
Results1
Dose modifications
- One patient did not receive BV as they were taken off the protocol at the treating physician’s discretion during prophase.
- Three patients received a dose reduction of BV to 1.2 mg/kg due to mucositis or elevated ALT.
- One of these patients was taken off the protocol after Cycle 3 at the treating physician’s discretion.
Toxicity
- 67 patients were evaluable for toxicity assessment.
- There were no toxic deaths, cases of progressive multifocal leukoencephalopathy syndrome, or cases of Grade 3/4 neuropathy.
- Grade 3/4 toxicities occurring in more than 5% of cycles included hematologic toxicities, mucositis, and febrile neutropenia (Table 2)
Table 2. Grade 3/4 adverse events*
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase. |
||
|
Adverse event, % |
Grade 3 |
Grade 4 |
|---|---|---|
|
Hematologic |
||
|
Anemia |
13.8 |
0.8 |
|
Neutropenia |
5.0 |
19.0 |
|
Thrombocytopenia |
5.5 |
8.5 |
|
Febrile neutropenia |
13.0 |
0.5 |
|
Nonhematologic |
||
|
Oral mucositis |
5.3 |
0.3 |
|
Anaphylaxis |
0.3 |
— |
|
Appendicitis |
0.3 |
— |
|
Catheter-related infections |
0.5 |
— |
|
Lung infection |
0.8 |
— |
|
Sepsis |
— |
0.3 |
|
Skin infection |
0.3 |
— |
|
Urinary tract infection |
0.5 |
— |
|
Other infections and infestations |
1.8 |
0.3 |
|
Laboratory |
||
|
ALT increase |
3.8 |
1.0 |
|
AST increase |
2.0 |
0.3 |
|
Hypokalemia |
3.3 |
0.3 |
|
Vascular |
||
|
Thromboembolic event |
0.8 |
— |
Response
- Relapse occurred in 14 patients (~20%) and were the only events contributing to event-free survival (EFS).
- 79% of relapses occurred within 10 months of diagnosis (median time from diagnosis to relapse, 7.5 months; range, 5.5–22 months).
- Only one patient relapsed during therapy (after the 6th chemotherapy cycle, but before off-therapy evaluation).
- Lung involvement was the only clinical factor associated with EFS (p = 0.026).
- Other response parameters can be seen in Table 3. The median follow-up for patients who were alive at last contact was 2.5 years (range, 1.2–5.2 years).
Table 3. Response*
|
CI, confidence interval; EFS, event-free survival; OS, overall survival. |
||
|
Response |
% |
95% CI |
|---|---|---|
|
2-year EFS |
79.1 |
67.2–87.1% |
|
2-year OS |
97.0 |
88.1–99.2% |
|
Complete response rate |
||
|
After Cycle 2 |
62 |
— |
|
After Cycle 6 |
97 |
— |
- Fifty-seven patients had available baseline data for the analysis of minimal disseminated disease (MDD), which was defined as >10 normalized copy numbers of the NPM-ALK fusion transcript.
- MDD was highly predictive of outcome and impacted the 2-year EFS (p = 0.00043) and overall survival (p = 0.041).
- The 2-year EFS estimate was 89.0% for MDD-negative patients (n = 38) vs 52.6% for MDD-positive patients (n = 19).
Conclusions
The addition of BV to standard chemotherapy for pediatric patients with newly diagnosed, non-localized, ALK+/CD30+ ALCL prevents relapses during therapy without adding significant toxicity. This is an important result for patients with ALCL, as those that relapse during treatment tend to have worse outcomes. The study also demonstrated that MDD detection in peripheral blood can be used as a prognostic tool for EFS. However, this study was limited in that patients were spread sparsely between treatment centers so inter-laboratory differences may have influenced the results. Therefore, further studies are required to test the efficacy of BV in different subgroups of patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

