All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
CAPTIVATE trial results show that 12 cycles of combined ibrutinib + venetoclax may allow treatment-free remission in patients with undetectable MRD
Ibrutinib, a Bruton’s tyrosine kinase inhibitor, and venetoclax, a BCL-2 inhibitor, are both approved in the US and Europe for the first-line treatment of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).1-4 Their effects are complimentary as ibrutinib is effective in shrinking lymph nodes, and venetoclax is effective in clearing blood and bone marrow of disease. Watch this video interview with William G. Wierda on the CAPTIVATE trial, here.
CAPTIVATE (NCT02910583) is an ongoing phase II study of ibrutinib plus venetoclax as a first-line therapy in patients with CLL and SLL. Patients were randomized based on the minimal residual disease (MRD) status following 12 cycles of combination therapy. William G. Wierda presented the primary endpoint results of this MRD cohort during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, and here, we are pleased to provide a summary of his talk.5
Study design
Patients deemed eligible based on the following criteria:
- < 70 years of age
- Previously untreated CLL/SLL
- Active disease requiring treatment per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria
- European Cooperative Oncology Group (ECOG) performance status 0–1
The primary endpoint was 1-year disease-free survival (DFS) rate in patients with confirmed undetectable MRD (uMRD) who were randomized to placebo versus ibrutinib to investigate if remission could be achieved without treatment in uMRD settings. DFS was defined as no MRD relapse (≥ 10−2 confirmed at two separate timepoints) and no disease progression or death. Key secondary endpoints included uMRD, response, progression-free survival (PFS), tumor lysis syndrome (TLS) risk reduction, and safety.
Treatments were given in 28-day cycles. All patients received ibrutinib for three cycles, and then underwent a TLS risk evaluation prior to the initiation of venetoclax. Study design is depicted in Figure 1.
Figure 1. Study design and patient disposition5
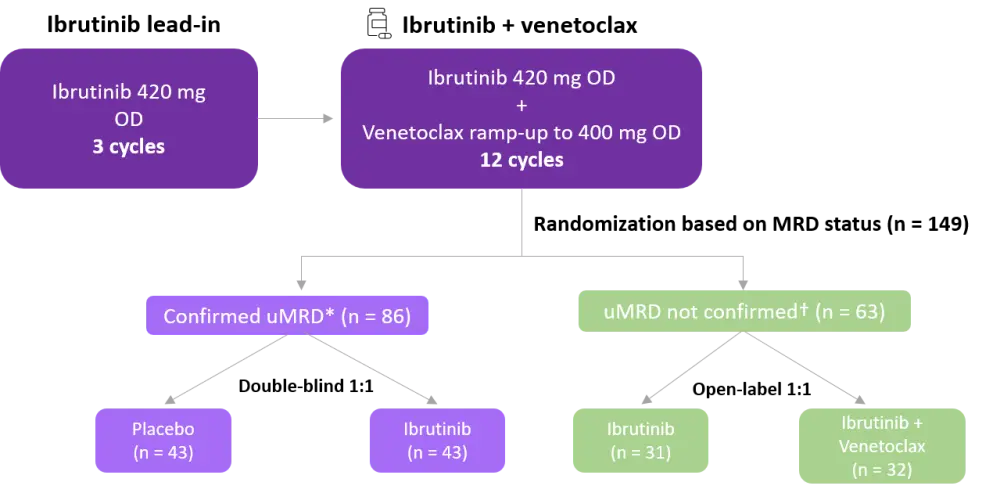
MRD, minimal residual disease; OD, once daily; uMRD, undetectable MRD.
*Defined as serial uMRD (< 10−4 by 8-color flow cytometry) over ≥ 3 cycles, uMRD in both peripheral blood (PB) and bone marrow (BM).
†Defined as detectable MRD, uMRD not confirmed serially, or not confirmed in both PB and BM.
Results
The number of patients included in the MRD cohort was 164 with a median age of 58 years (range, 28–69 years). Patient characteristics are given in Table 1.
Table 1. Baseline characteristics in the MRD cohort5
|
ALC, absolute lymphocyte count; IGHV, immunoglobulin heavy chain variable region. |
|
|
Characteristic |
N = 164 |
|---|---|
|
Rai stage III/IV disease, n (%) |
53 (32) |
|
High-risk characteristics, n (%) Del(17p)/TP53 mutation Del(11q) Complex karyotype Unmutated IGHV |
32 (20) 28 (17) 31 (19) 99 (60) |
|
Any cytopenia, n (%) |
59 (36) |
|
Lymph node diameter ≥ 5 cm, n (%) |
53 (32) |
|
Median ALC × 109/L, range ALC ≥ 25 × 109/L, n (%) |
56 (1–419) 125 (76) |
Following the ibrutinib lead-in phase, the TLS risk was reduced to medium or low in most patients with high-risk for TLS at baseline. This phase also reduced the number of patients requiring hospitalization for venetoclax initiation; 82% of patients could start venetoclax treatment without hospitalization.
Figure 2 shows the uMRD rates after 12 cycles of combination treatment among different groups.
Figure 2. uMRD rates5
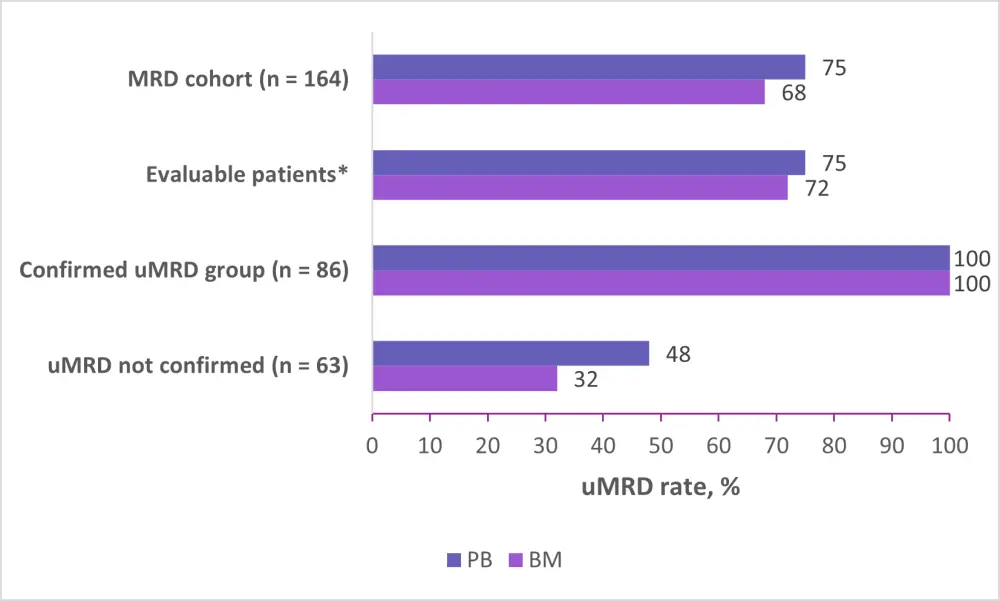
BM, bone marrow; MRD, minimal residual disease; PB, peripheral blood; uMRD, undetectable MRD.
*n = 163 for PB; n = 155 for BM.
Almost all patients with uMRD in PB also had uMRD in BM (93%).
MRD-guided randomization arms
The number of patients who were eligible for randomization based on MRD status was 149 (Figure 1), and patients were stratified by immunoglobulin heavy chain variable region (IGHV) gene mutation status. Fifteen patients discontinued ibrutinib lead-in or combination treatment due to disease progression, adverse events (AEs), patient withdrawal or investigator decision.
Baseline characteristics among randomization arms are summarized in Table 2.
Table 2. Baseline characteristics by randomization arms5
|
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; IGHV, immunoglobulin heavy chain variable region; uMRD, undetectable minimal residual disease |
||||
|
Characteristic |
Confirmed uMRD |
Not confirmed uMRD |
||
|---|---|---|---|---|
|
Placebo |
Ibrutinib |
Ibrutinib |
Ibrutinib + |
|
|
Median age, years (range) |
61 (43–69) |
56 (34–69) |
58 (28–69) |
56 (37–69) |
|
Rai stage III/IV disease, n (%) |
15 (35) |
8 (19) |
14 (45) |
11 (34) |
|
High-risk characteristics, n (%) Del(17p)/TP53 mutation Del(11q) Complex karyotype Unmutated IGHV |
2 (5) 8 (19) 4 (9) 30 (70) |
13 (30) 10 (23) 13 (30) 30 (70) |
5 (16) 3 (10) 5 (16) 14 (45) |
8 (25) 2 (6) 4 (13) 15 (47) |
|
Any cytopenia, n (%) ANC ≤ 1.5 × 109/L Hemoglobin ≤ 11 g/dL Platelets ≤ 100 × 109/L |
19 (44) 5 (12) 14 (33) 4 (9) |
6 (14) 0 2 (5) 4 (9) |
13 (42) 2 (6) 9 (29) 9 (29) |
14 (44) 4 (13) 7 (22) 9 (28) |
|
Lymph node diameter ≥ 5 cm, n (%) |
18 (42) |
10 (23) |
7 (23) |
11 (34) |
|
Median ALC × 109/L, range ALC ≥ 25 × 109/L, n (%) |
53 (1–235) 32 (75) |
56 (2–256) 34 (79) |
85 (1–342) 25 (81) |
87 (3–419) 24 (75) |
The median follow-up after randomization was 16.6 months. In the confirmed uMRD arm, 1-year DFS rate was 95.3% (%95 CI, 82.7–98.8) with placebo and 100.0% (%95 CI, 100.0–100.0) with ibrutinib (p = 0.15).
The median follow-up on the study was 31.3 months, and 30-month PFS rate was 95.3% (95% CI, 90.4–97.8) in the MRD cohort (n = 164).
30-month PFS rates (95% CI) by randomization arm
- Confirmed uMRD
- Placebo: 95.3% (82.7–98.8)
- Ibrutinib: 100.0% (100.0–100.0)
- Not confirmed uMRD
- Ibrutinib: 95.2% (70.7–99.3)
- Ibrutinib + venetoclax: 96.7% (78.6–99.5)
In the uMRD not confirmed arm, uMRD rates following subsequent treatment with ibrutinib or ibrutinib + venetoclax are shown in Figure 3. Continued ibrutinib + venetoclax treatment produced greater increases in uMRD rates compared with ibrutinib alone.
Figure 3. uMRD rates in the uMRD not confirmed arm5
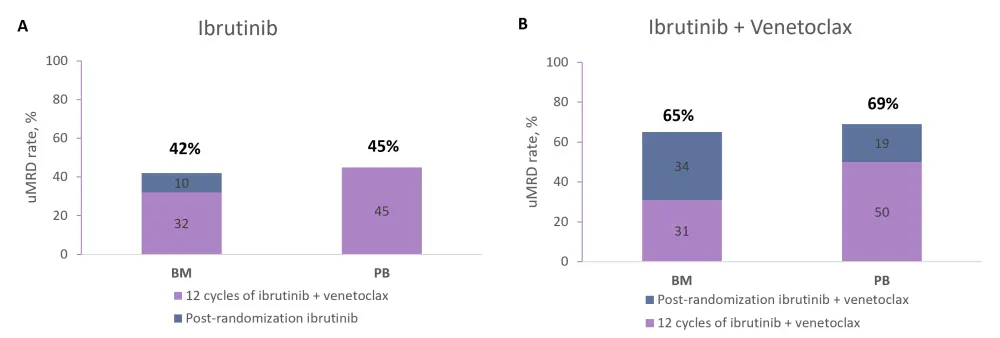
A uMRD rates after 12 cycles of ibrutinib + venetoclax treatment and subsequent ibrutinib treatment, and B uMRD rates after 12 cycles of ibrutinib + venetoclax treatment and subsequent ibrutinib + venetoclax treatment.
BM, bone marrow; PB, peripheral blood; uMRD, undetectable minimal residual disease.
Safety
AEs of interest included diarrhea, arthralgia, hypertension, atrial fibrillation, bleeding, neutropenia, and infections, which were more prevalent during the first six cycles of ibrutinib + venetoclax treatment before randomization. The prevalence decreased over time independent of MRD-guided randomization treatment. The AEs leading dose reduction or discontinuation were infrequent.
The frequency of Grade ≥ 3 AEs was low, the most common being neutropenia, hypertension, thrombocytopenia, and diarrhea.
Conclusion
The high rate of 1-year DFS with placebo (95%) in patients with uMRD following 12 cycles of ibrutinib + venetoclax may justify a fixed-duration treatment approach with this combination. Safety analysis did not produce any new safety concerns, and AEs appeared to decrease after the first six cycles of ibrutinib + venetoclax. This study demonstrated that the combination of ibrutinib and venetoclax may produce deep MRD remission in both BM and PB with a once-daily, oral, chemotherapy-free, fixed-duration regimen in the first-line treatment of patients with CLL. During the 62nd ASH Annual Meeting and Exposition presentation, the authors acknowledged that additional data were required to further evaluate the clinical value of the study findings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

