All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Consolidative HDC-ASCT versus non-myeloablative immunochemotherapy for patients with primary CNS lymphoma
Currently, high-dose methotrexate (HD-MTX) based induction immunochemotherapy is prescribed to treat patients with primary central nervous system lymphoma (PCNSL) who qualify for intensive treatment approaches. This is followed by consolidative high-dose chemotherapy and autologous stem cell transplantation (HDC-ASCT). However, it is unclear whether consolidation with conventional-dose non-myeloablative immunochemotherapy, using cytotoxic agents that can cross the blood-brain barrier, can also defeat chemoresistance and subsequently eradicate minimal residual disease.1
Here, we report primary results from the phase III MATRix/IELSG43 (NCT02531841) trial comparing HDC-ASCT with non-myeloablative consolidation therapy in patients with PCNSL, presented by Gerald Illerhaus at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition in December 2022 as part of the late-breaking abstracts session.1
Study design
This was an open-label, randomized phase III trial conducted in 79 centers across five European countries (Germany, Italy, Denmark, Norway, and Switzerland). Included patients were newly diagnosed with PCNSL, HIV-negative, aged 18–65 years irrespective of Eastern Cooperative Oncology Group (ECOG) Performance Status or 66–70 years with ECOG Performance Status ≤2, and had adequate organ function.
- induction consisted of four cycles of the MATRix regimen (rituximab: 375 mg/m2/d on Days 0 and 4; methotrexate: 3.5 g/m2 on Day 1; cytarabine: 2 × 2 g/m2/d on Days 2 and 3; thiotepa: 30 mg/m2 on Day 4; every 21 days)
- stem cell harvest was conducted after the 2nd cycle
- patients achieving at least partial response after completion of induction were randomly allocated to
- Arm A: two cycles of R-DeVIC regimen (rituximab: 375 mg/m2 on Day 0; dexamethasone: 40 mg/d on Days 1–3; etoposide: 100 mg/m2/d on Days 1–3; ifosfamide: 1,500 mg/m2/d on Days 1–3; carboplatin: 300 mg/m2 on Day 1); or
- Arm B: HDC (BCNU 400 mg/m2 on Day 6 and thiotepa 2 × 5 mg/kg/d on Days 5 and 4) followed by ASCT on Day 0.
The primary endpoint was progression-free survival (PFS) and key secondary efficacy endpoints included complete response, overall survival, quality of life, and safety (toxicity and neurotoxicity)
Results
- Between July 2014 and August 2019, 368 patients were registered of which 346 started treatment, 260 (75%) completed the induction therapy, and 115 and 114 patients were randomly assigned to arm A and arm B, respectively
- Toxicities (25%) and disease progression (10%) were the primary reasons for not reaching randomization
- The baseline characteristics were well balanced between arms
- The median age of the randomized patients was 59 years, with 22.3% of patients aged ≥65 years
Efficacy
- At a median follow-up of 44 months, 69% of patients responded to induction treatment, 27% achieved complete remission, and 52% achieved partial remission.
- Both consolidation strategies were well tolerated, with 87% and 97% of the patients completing treatments in arm A and arm B, respectively.
- Overall, 13 patients died of treatment-related complications during induction treatment, 11 of them due to neutropenic infectious complications.
- Consolidation treatment with R-DEVIC or HDC-ASCT resulted in a substantial increase of patients with complete response (65% and 68% in arm A and arm B, respectively; p = 0.71).
- Six patients died of toxicity during consolidation treatment (two patents in arm A and four patients in arm B) and six patients died of unrelated causes while relapse-free (five patents in arm A and one patient in arm B).
- The 3-year PFS and 3-year overall survival differed significantly between both the arms (p = 0.0003 and p = 0.01, respectively; Figure 1).
Results of the subgroup analysis for PFS favored arm B compared with arm A.
Figure 1. Three-year PFS and OS in arm A and arm B*
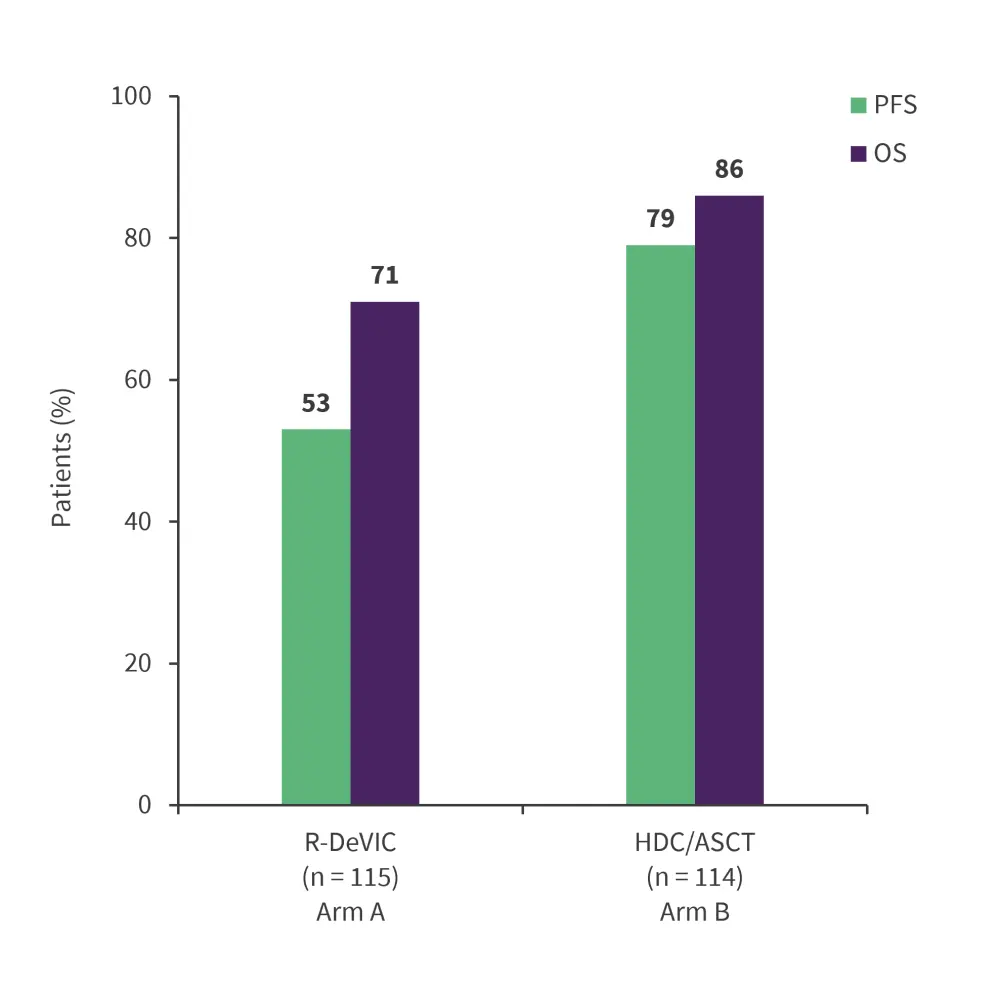
HDC/ASCT, high-dose chemotherapy and autologous stem cell transplantation; OS, overall survival; PFS, progression-free survival; R-DeVIC, rituximab-dexamethasone + etoposide + ifosfamide + carboplatin.
*Adapted from Illerhaus, et al.1
Toxicity
- safety was comparable across both arms
- no difference in toxicity was observed between both arms (Table 1)
- four treatment-related deaths were reported in arm B versus no deaths in arm A
Table 1. Grade 3 and 4 toxicities in arm A and arm B*
|
HDC/ASCT, high-dose chemotherapy and autologous stem cell transplantation; R-DeVIC, rituximab-dexamethasone + etoposide + ifosfamide + carboplatin. |
||
|
Toxicity, % |
R-DeVIC |
HDC/ASCT |
|---|---|---|
|
Neutropenia |
56 |
75 |
|
Thrombocytopenia |
83 |
95 |
|
Anemia |
69 |
75 |
|
Febrile neutropenia/Infections |
15 |
63 |
|
Infections |
14 |
53 |
|
Oral mucositis |
0 |
55 |
|
Vascular disorder |
3 |
9 |
|
Nervous system disorders |
5 |
5 |
|
Psychiatric disorders |
0 |
5 |
|
Cardiac disorders |
0 |
3 |
|
Renal toxicity |
0 |
5 |
Conclusion
In this study, consolidation with HDC-ASCT resulted in improved outcomes with a very good risk-to-benefit ratio vs non-myeloablative chemoimmunotherapy in patients with PCNSL. Additionally, there were no detectable adverse effects on neurocognitive function. These results support the standard use of consolidation HDC-ASCT therapy over non-myeloablative chemoimmunotherapy for fit patients with PCNSL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

