All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
DZD4205, an oral JAK inhibitor for the treatment of R/R PTCL: JACKPOT8 phase I trial results
Featured:
Relapsed or refractory (R/R) peripheral T-cell lymphoma (PTCL) is an aggressive and rare type of non-Hodgkin lymphoma. The prognosis of PTCL is poor, with a 5-year overall survival rate of <30% and a median progression-free survival of <4 months. A consensus regarding standard therapy for PTCL has not yet been reached and the current therapies approved have shown limited benefits, with objective response rates (ORRs) of <30%. Therefore, there is a clinical unmet need for this patient population.1
The JAK-STAT pathway is one of the major intracellular signaling pathways that regulates cellular immunity, proliferation, differentiation, apoptosis, and oncogenesis; this pathway also plays an important role in the pathogenesis of PTCL. DZD4205 (previously known as AZD4205) is a potent, highly selective, second-generation JAK1 inhibitor that has demonstrated antitumor activity in lymphoma cell lines in vitro and in tumor xenografts in vivo. Therefore, DZD4025 may be a potential therapeutic option for this unmet clinical need.1
During the 16th International Conference on Malignant Lymphoma (16-ICML), Won Seog Kim presented results of the ongoing phase I/II JACKPOT8 study (NCT04105010), which investigates DZD4205 for the treatment of patients with R/R PTCL.1 The results are summarized below.
Study design and patient characteristics1
- A two-part, open label, single arm study of DZD4205 in R/R PTCL:
- Part A: a dose escalation with DZD4025 administered at 150 mg or 250 mg once daily
- Part B: a dose expansion based on Part A
- Primary objective: to assess the safety and tolerability of DZD4205
- Secondary objectives: to assess the pharmacokinetics and antitumor efficacy of DZD4205
- Safety and efficacy were assessed according to Common Terminology Criteria for Adverse Events version 5.0 and 2014 Lugano classification, respectively
- Data cut-off: December 7, 2020
- Enrollment: N = 47 (Cohort 1: 150 mg once daily, n = 31; Cohort 2: 250 mg once daily, n = 16)
- Patient characteristics can be seen in Table 1
- The total cohort had a median age of 62 years, and a median of two prior lines of therapy
- Of note, there was a higher proportion of males (68.1% of the total cohort)
Table 1. Patient characteristics*
|
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; BM, bone marrow; ECOG, Eastern Cooperative Oncology Group; HDAC, Histone deacetylase; HSCT, hematopoietic stem cell transplantation; MEITL, monomorphic epitheliotropic intestinal T-cell Lymphoma; NK/TCL, natural killer/T-cell lymphoma; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma. |
|||
|
Characteristic, % (unless otherwise stated) |
Total |
Cohort 1: 150 mg |
Cohort 2: 250 mg |
|---|---|---|---|
|
Median age (range), years |
62.0 (29–79) |
63.0 (33–78) |
61.5 (29–79) |
|
Female/male |
31.9/68.1 |
38.7/61.3 |
18.7/81.3 |
|
ECOG Performance Status |
|
|
|
|
0 |
53.2 |
51.6 |
56.3 |
|
1 |
47.8 |
48.4 |
43.7 |
|
Prior regimens |
|
|
|
|
Median number (range) |
2 (1–8) |
2 (1–8) |
2.5 (1–8) |
|
≥3 lines |
40.4 |
35.5 |
50.0 |
|
Chemotherapy |
100 |
100 |
100 |
|
HDAC inhibitor |
23.4 |
16.1 |
37.5 |
|
CD30 targeting therapy |
4.3 |
6.5 |
0.0 |
|
PTCL subtype |
|
|
|
|
PTCL-NOS |
42.6 |
45.2 |
37.5 |
|
AITL |
42.6 |
45.2 |
37.5 |
|
Extra-nodal nasal NK/TCL |
6.4 |
3.2 |
12.5 |
|
ALCL ALK-negative |
6.4 |
6.5 |
6.3 |
|
MEITL |
2.1 |
0.0 |
6.3 |
|
BM involvement at baseline |
34.0 |
35.5 |
31.3 |
|
Prior HSCT |
12.8 |
16.1 |
6.3 |
Results1
Efficacy
ORRs for the 42 patients who had completed ≥1 posttreatment antitumor efficacy assessment can be seen in Figure 1.
- The total cohort ORR was 47.6%, comprised of a complete response rate of 19% and a partial response rate of 28.6%.
- ORR was slightly higher in Cohort 1 than Cohort 2 (51.9% vs 40.0%), and Cohort 1 had a complete response rate of 22.2%.
Figure 1. Best overall response rate*
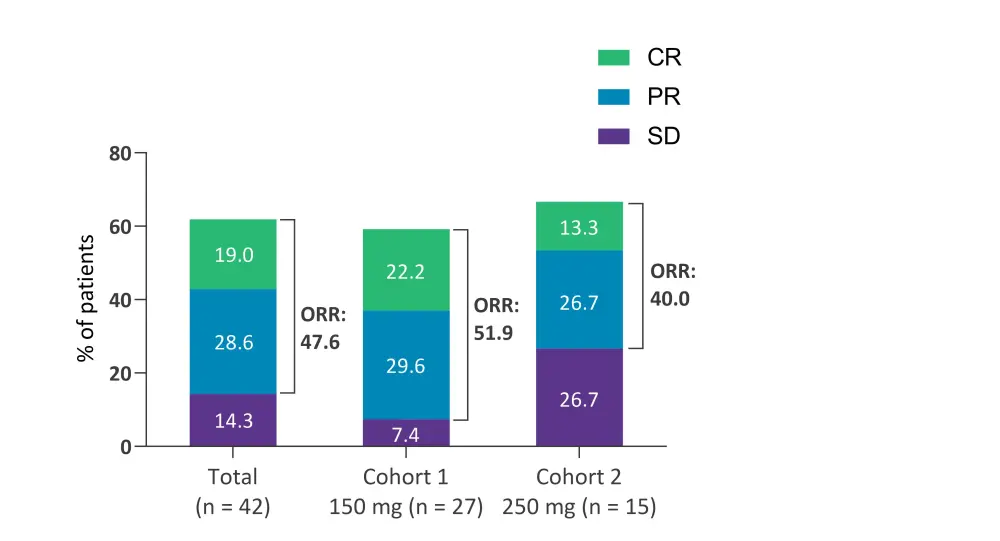
CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease.
*Data from Kim, et al.1
There was a significant reduction in tumor burden in two-thirds of the total cohort. The median duration of response was not yet reached; however, the longest duration of response was >12 months.
Highest ORR were seen for anaplastic large-cell lymphoma, anaplastic lymphoma kinase-negative, and angioimmunoblastic T-cell lymphoma subtypes (66.7% and 63.3% of patients respectively). The ORR by histological subtype can be seen in Figure 2.
Figure 1. Best ORR by histological subtype*
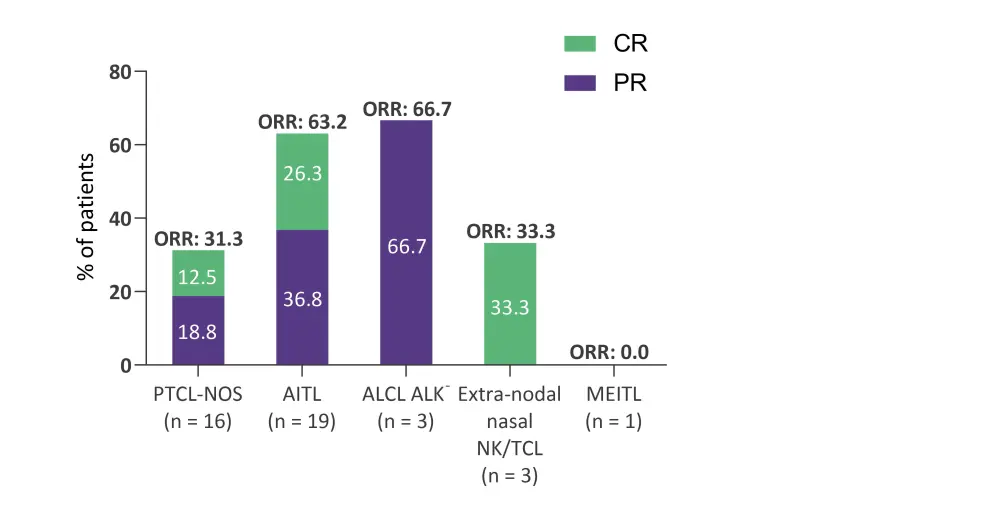
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ALK-, anaplastic lymphoma kinase-negative; CR, complete response; MEITL, monomorphic epitheliotropic intestinal T-cell Lymphoma; NK/TCL, natural killer/T-cell lymphoma; ORR, overall response rate; PR, partial response; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified.
Safety and tolerability
Treatment emergent adverse events (TEAEs) can be seen in Table 2.
- In total, 87.2% of patients experienced ≥1 TEAE; with a higher percentage occurring in Cohort 2 compared with Cohort 1 (93.8% vs 83.9%, respectively).
- Overall, 34% of patients experienced ≥1 serious TEAE.
- Dose reduction and discontinuation occurred in 27.7% and 10.6% of patients respectively.
Table 2. TEAEs in each cohort*
|
TEAE, treatment emergent adverse event. |
|||
|
TEAEs, % |
Total |
Cohort 1: 150 mg |
Cohort 2: 250 mg |
|---|---|---|---|
|
≥1 TEAE |
87.2 |
83.9 |
93.8 |
|
≥1 Grade 3 TEAE |
51.1 |
48.4 |
56.3 |
|
≥1 serious TEAE |
34.0 |
29.0 |
43.8 |
|
≥1 TEAE leading to dose reduction |
27.7 |
19.4 |
43.8 |
|
≥1 TEAE leading to dose discontinuation |
10.6 |
12.9 |
6.3 |
Grade ≥3 TEAEs can be seen in Table 3.
- The most common Grade ≥3 TEAEs were neutropenia (23.4%), thrombocytopenia (17.0%), and pneumonia (12.8%).
- The majority of TEAEs were manageable and reversable.
Table 3. Grade ≥3 TEAEs occurring in >10% of patients*
|
TEAE, treatment emergent adverse event. |
|||
|
TEAE, % |
Total |
Cohort 1: 150 mg |
Cohort 2: 250 mg |
|---|---|---|---|
|
Neutropenia |
23.4 |
25.8 |
18.8 |
|
Thrombocytopenia |
17.0 |
12.9 |
25.0 |
|
Pneumonia |
12.8 |
12.9 |
12.5 |
|
Anemia |
6.4 |
9.7 |
0.0 |
|
Hepatic enzyme increased |
6.4 |
9.7 |
0.0 |
Conclusion
DZD4205 showed promising antitumor activity in patients with R/R PTCL and it was well tolerated; the safety profile was comparable with currently approved therapies, and the majority of TEAEs were manageable and reversable. The 150 mg once-daily dose was selected as the recommended phase II dose, as it was deemed to be more efficacious and had less TEAEs compared with the higher dose. The phase II portion of the trial has been initiated.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Sumayya Khan
Sumayya Khan