All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Educational theme | CSF analysis of ctDNA for lymphoma with CNS involvement
This article is part of our educational series on the role of circulating tumor DNA (ctDNA) in the management of patients with lymphoma.
Central nervous system (CNS) involvement in aggressive lymphomas is associated with very poor prognosis and remains a major diagnostic and prognostic challenge. Diagnosis of CNS involvement usually requires an invasive brain biopsy, or cytology and flow cytometry of the cerebrospinal fluid (CSF). However, the sensitivity of these assays remains low. More recently, circulating tumor (ctDNA) in the CSF is being investigated as a prognostic tool for CNS progression.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, two abstracts were presented on CSF analysis of ctDNA for lymphoma with CNS involvement.1,2 These were previewed in our pre-ASH summary article and are covered in more detail below.
Xiaoxiao Wang and colleagues assessed the correlation between CSF ctDNA and CNS involvement in 67 patients with diffuse large B-cell lymphoma (DLBCL) who were considered as high-risk for CNS relapse according to the CNS-International Prognostic Index (CNS-IPI).1 Targeted mutational profiling using a validated panel of > 400 genes was performed on CSF- and plasma-derived ctDNA with matched systemic tumor tissues. CSF ctDNA concentration, but not plasma ctDNA concentration, was found to correlate with a high CNS-IPI score.
Gene alterations were assessed in 20 patients that had matched ctDNA samples from the tissue, plasma, and CSF. This analysis revealed 48 unique gene alterations in the CNS, compared with the tissue; of which 24 were completely unique to the CSF, and 24 were shared with those found in the plasma (Figure 1). These alterations were enriched in genes involved in epigenetic regulation, cell cycle/apoptosis, and immunity. 1
Figure 1. Gene alterations in each tissue type1
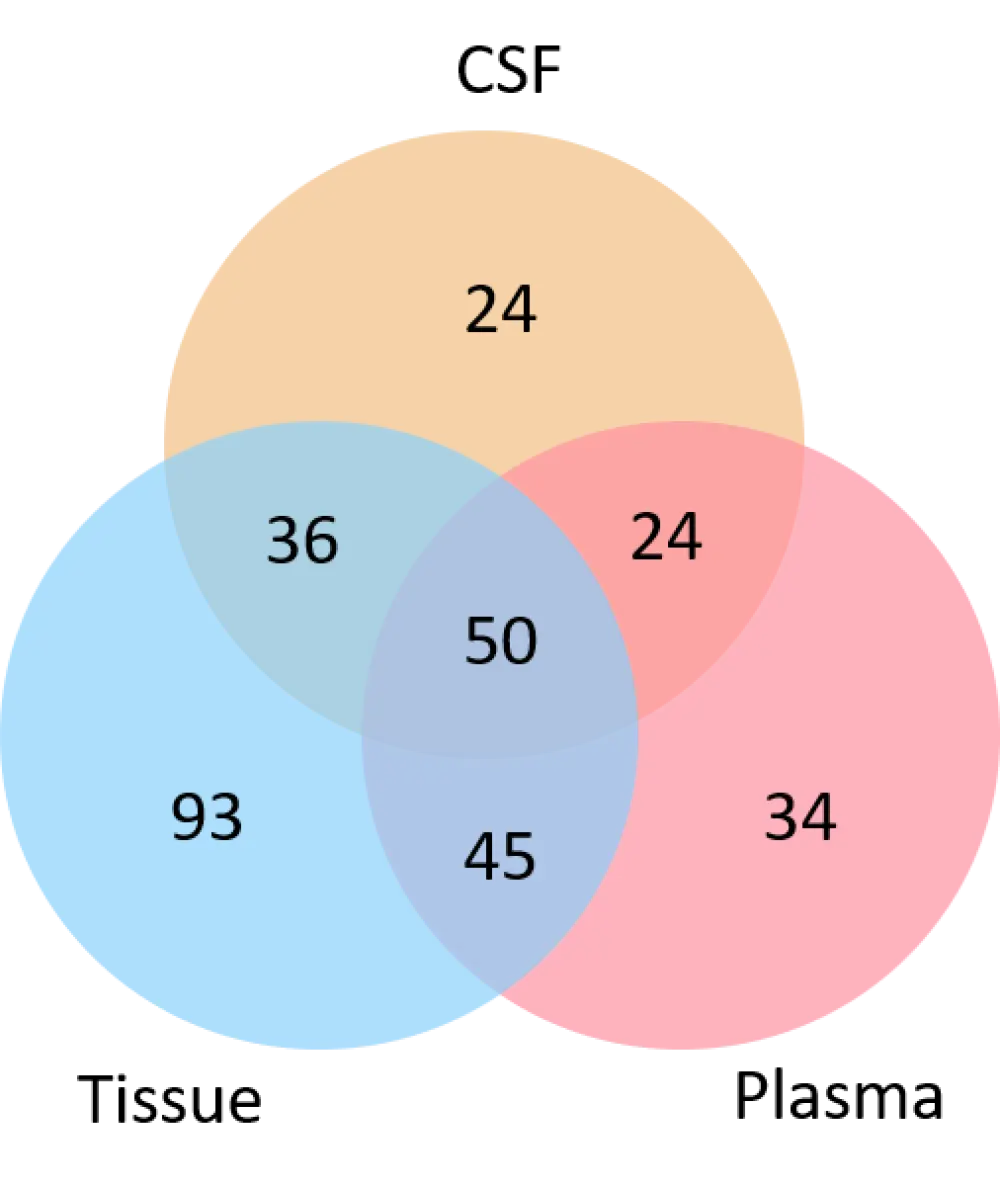
CSF, cerebrospinal fluid.
To screen for CNS-related molecular features in CSF, gene alterations in brain tumor tissue and CSF from 10 patients with primary CNS lymphoma (PCNSL) were sequenced and compared. Aberrations in 13 genes were common in both brain tissue and CSF; aberrations in five of these genes, BTG2, PIM1, DUSP2, ETV6, and CXCR4, were identified in more than 20% of patients. Seven out of 10 patients with high-risk DLBCL who were later confirmed to have CNS involvement by MRI and flow cytometry, also had multiple aberrations in these five genes, suggesting that ctDNA of these five genes could potentially be used as biomarkers for CNS involvement, though this requires further investigation.1
Adam Olszewski and colleagues investigated CSF ctDNA as a prognostic and diagnostic tool for CNS recurrence in aggressive lymphomas.2 They used a novel next-generation sequencing (NGS)-based minimal residual disease (MRD) assay, whereby a patient’s primary tumor is analyzed by NGS to identify unique tumor-specific clonotypes from rearrangements of the variable, diversity, and joining (VDJ) regions of the immunoglobulin gene loci. These tumor-specific clonotypes were then tracked in CSF and plasma samples. This assay allows for the quantification of the amount of clonotypic DNA and the frequency of the clonotype against the background of all other B cells in the sample.
The diagnostic sensitivity of the NGS-MRD assay was examined in the CSF of a prospective cohort of six patients with lymphoma with known parenchymal or leptomeningeal CNS involvement. ctDNA was detected in 100% of patients including those who had a negative cytology or flow cytometry results. The median ctDNA copy count and frequency for parenchymal and leptomeningeal CNS lymphomas can be seen in Table 1. ctDNA was also detectable in historic CSF samples from six patients with CNS involvement with either a negative PCR for immunoglobulin heavy chain rearrangement, or insufficient DNA for PCR analysis.2
Table 1. ctDNA quantification and frequency in CSF of patients with lymphoma with CNS involvement2
|
CNS, central nervous system; ctDNA, circulating tumor deoxyribonucleic acid. |
||
|
ctDNA quantification |
Parenchymal CNS involvement |
Leptomeningeal CNS involvement |
|---|---|---|
|
Median ctDNA copy count /mL (range) |
2 (0.4–929) |
2,233 (1.2–5620) |
|
Median clonotype frequency, % (range) |
9 (0.03–68.9) |
37 (1.7–98.4) |
A prospective study of 19 newly diagnosed patients with high-risk lymphomas (DLBCL, high-grade B-cell lymphoma/double hit lymphoma, B-cell acute lymphoblastic leukemia, Burkitt lymphoma, and plasmablastic lymphoma) with no known CNS involvement was then performed to examine the prognostic value of the NGS-MRD assay, by using CSF collected at diagnosis. The assay was positive for tumor-specific clonotypes in the CSF of 42% of patients. The median ctDNA copy count was 3/mL (range, 0.9–15.6) and the median clonotype frequency was 18.6% (range, 1.4–28.5%). In these patients, no significant correlation was observed between the amount of ctDNA and the number of red blood cells in the CSF, therefore ruling out possible contamination by blood as a source for the ctDNA.2
All 19 patients received standard chemotherapy and CNS prophylaxis according to their histology. With a median follow-up of 15 months, two patients experienced CNS recurrence. Both patients were positive for the NGS-MRD assay. The estimated 1-year risk with a positive NGS-MRD assay was 29% (95% CI, 8–74) compared with 0% for a negative NGS-MRD assay (p = 0.07). This study therefore demonstrates a 100% sensitivity and a 100% negative predictive value for the risk of future CNS recurrence; however, the small sample size warrants further investigation.2
Conclusions
Taken together, these studies highlight the importance of CSF ctDNA as a potential prognostic and diagnostic tool for assessing CNS involvement in lymphoma. Wang and colleagues demonstrated an association between CSF ctDNA concentration and CNS-IPI score in DLBCL. Aberrations in the five genes associated with a higher risk may also highlight patient populations that would benefit from prophylaxis treatment. The NGS-MRD assay used by Olszewski and colleagues provides a greater sensitivity than current CSF evaluations used to detect CNS involvement in patients with aggressive lymphoma (cytology, flow cytometry, and PCR). Earlier detection of CNS involvement by CSF ctDNA analysis may allow for adapted treatment and therefore a better prognosis for this patient population.
For other articles in this theme, click on the links below:
The role of ctDNA in lymphoma management
Monitoring treatment response in lymphoma using cfDNA
5hmC profiles of cfDNA predict R-CHOP treatment response in patients with DLBCL
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

