All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
EHA-SWG 2017 | Rare Lymphomas: advances in biology of MCL
On March 10–12 2017, the EHA-SWG meeting on Rare Lymphomas took place in Barcelona, Spain, and was jointly chaired by Prof. Martin Dreyling, from Klinikum der Universität München, Germany, and Prof. Marie José Kersten, from the Academic Medical Center, Amsterdam, The Netherlands.
On March 10th 2017, Elias Campo, from the Hospital Clínic, University of Barcelona, Spain, gave a talk titled “Advances in Biology”, during the “Mantle Cell Lymphoma” scientific session.
Elias Campo began by stating that MCL is thought of as an aggressive mature B-cell lymphoma. The primary oncogenic event is the t(11;14) translocation which deregulates cyclin D1. This is then followed by progressive changes in regulation of the cell cycle and DNA damage response, and activation of cell survival.
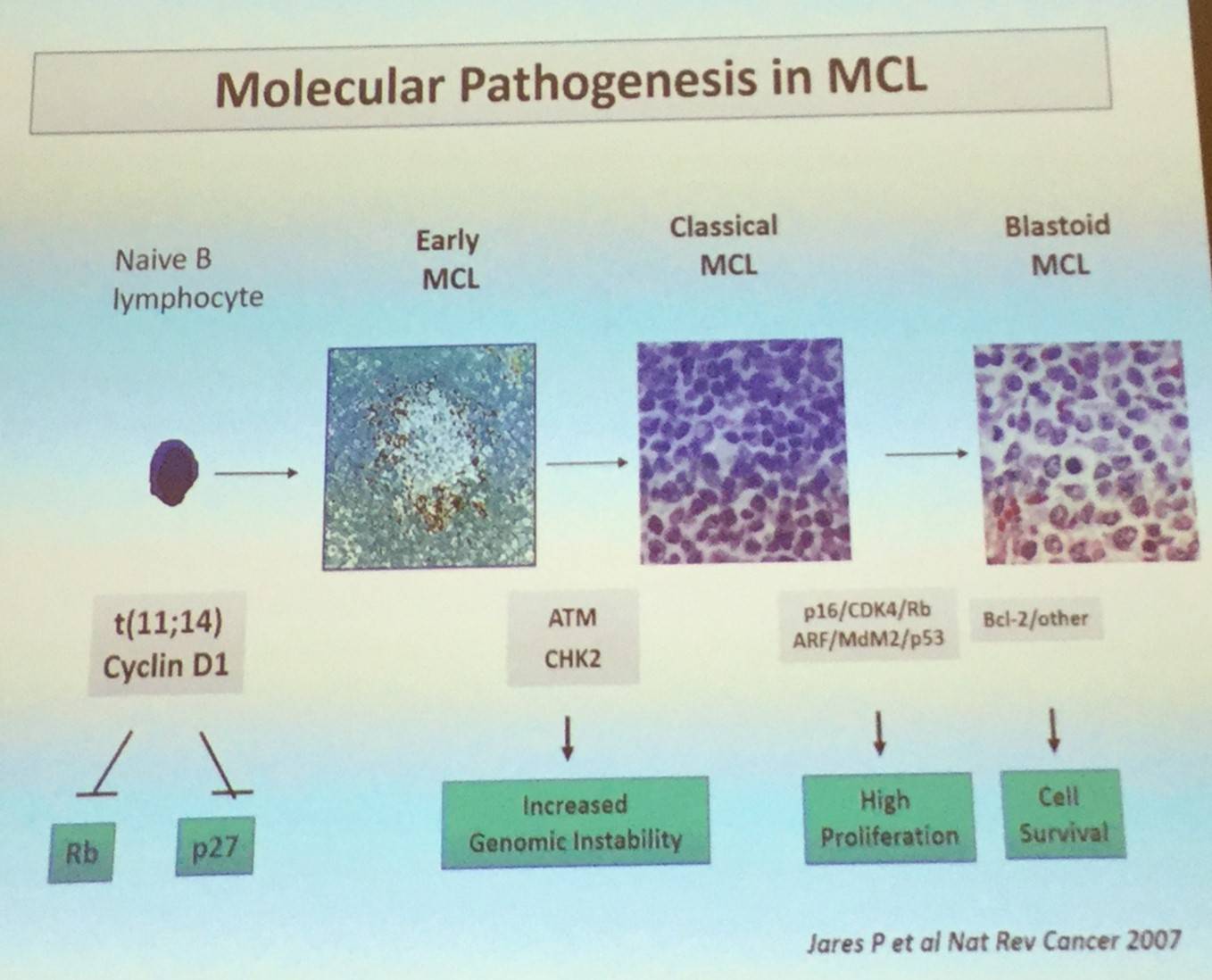
However, the discovery of CCND1-negative cases or unexpected indolent cases have tested this basic model, as well as broadening our understanding of the clinical heterogeneity of MCL.
MCL with indolent clinical behavior display “in situ” lesions and Mantle Zone pattern, low proliferation fraction, and are generally early stage disease. They have frequently been identified in retrospective biopsies of MCL patients, and the risk of progression to lymphoma appears low (1/15 cases with >1 year follow-up).
CCND1-negative MCL is identical in terms of morphology and pathology to conventional MCL but lacks the t(11;14) translocation. These cases tend to express the transcription factor SOX11 which can be identified in routine practice by immunohistochemistry. In over half of cases (55%), CCND2 translocation has been reported. Clinical presentation and evolution of CCND1-negative MCL is also similar to conventional MCL.
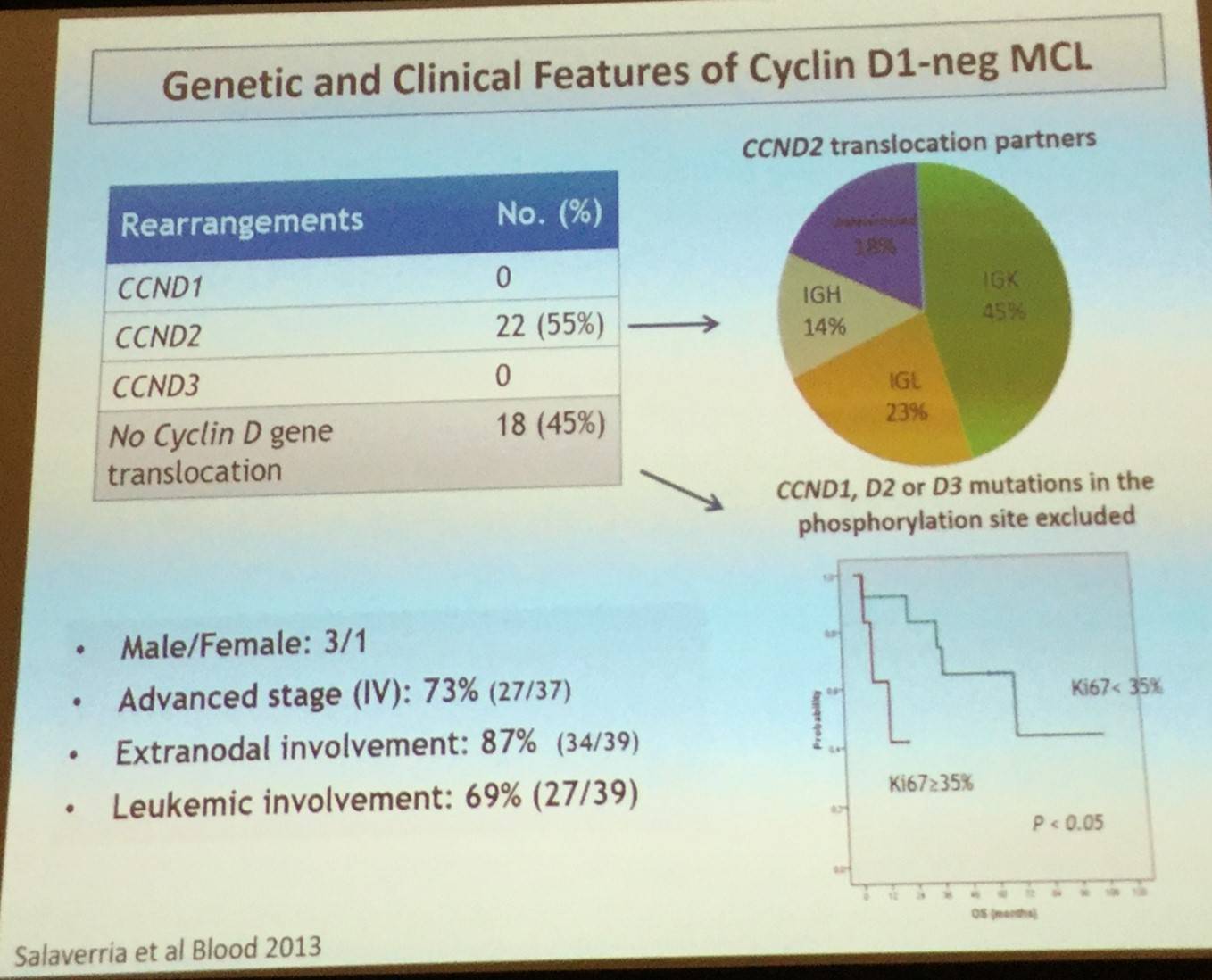
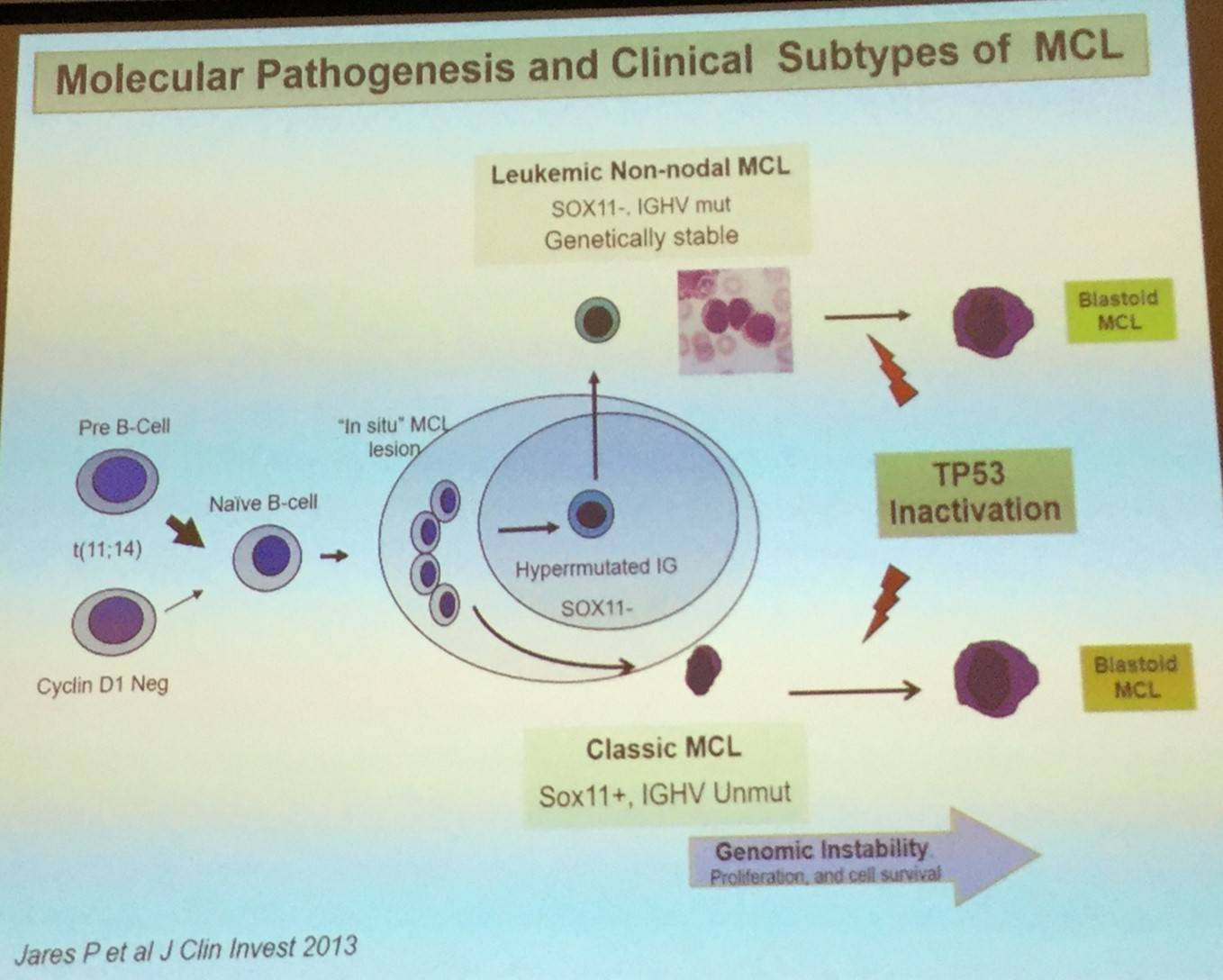
In addition to CCND1-negative MCL, two major biological subtypes of the disease have been recognized: classical MCL and leukemia non-nodal MCL.
Conventional MCL originates in a B-cell with little or no exposure to the germinal center microenvironment, has no or very low number of IGHV somatic mutations, develops increasing chromosomal instability, and clinically has a tendency for nodal dissemination and an aggressive evolution.
Leukemic non-nodal MCL, which has been recognized in the updated 2016 WHO classification, appears to derive from a cell which has been exposed to the germinal center microenvironment, contains a high number of IGHV somatic mutations, carries stable karyotypes, and clinically tends to present with a leukemic non-nodal disease and a prolonged indolent course.
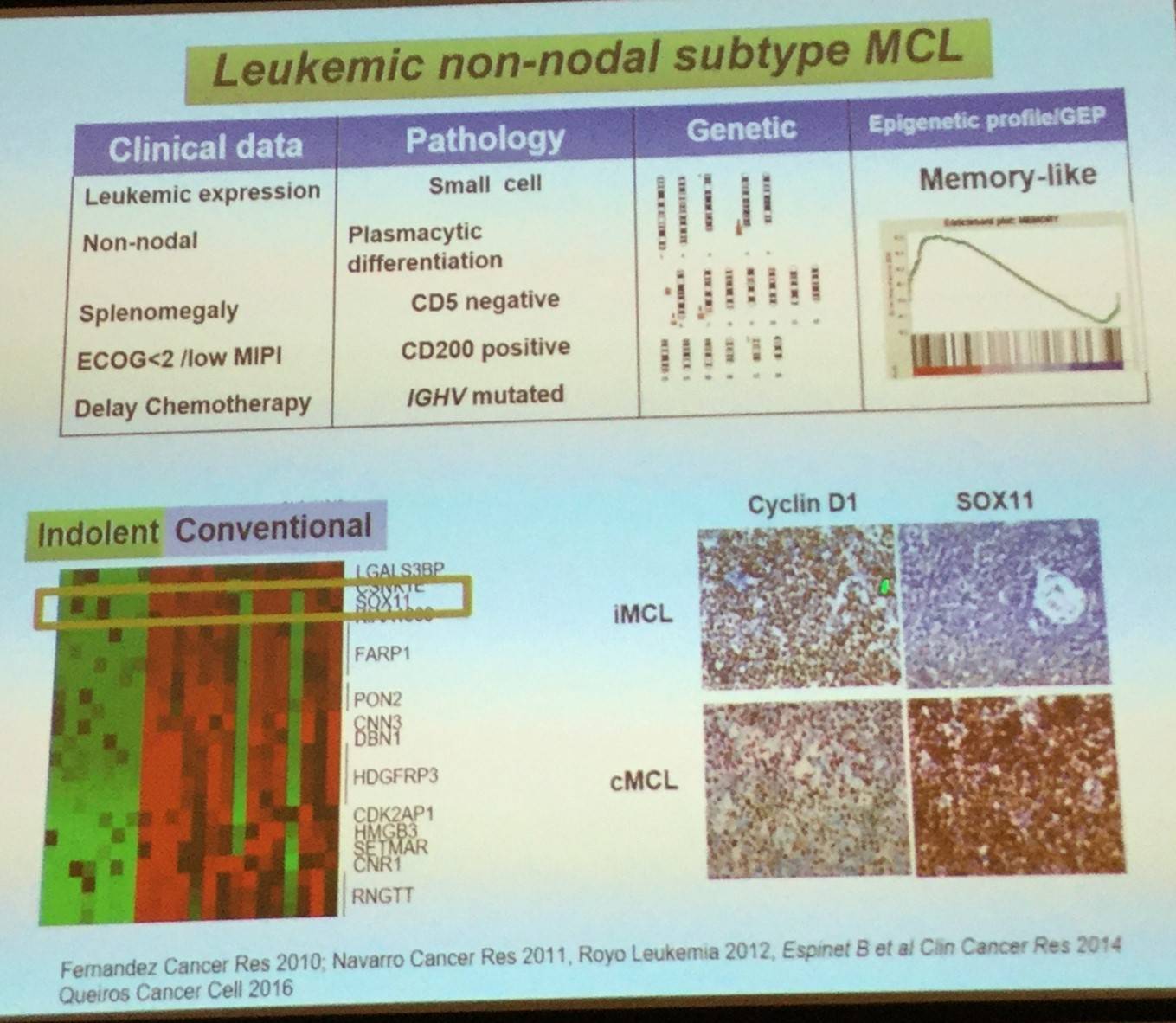
Elias Campo went on to explain that expression of the transcription factor SOX11 can be used to determine the pathogenesis of the two MCL subtypes: classical MCL (including CCND1-negative cases) highly express SOX11, whereas leukemic non-nodal MCL cases have very little or no expression of SOX11.
Global analysis of the methylation profile of MCL has found two subgroups whose epigenetic signature either related to naïve or memory B-cells as potential cell of origin. The two subgroups also seem to correspond with classical and leukemic non-nodal subtypes discussed above, and so these two subtypes of MCL may also differ in their epigenetic features. Most changed in methylation between normal cell of origin and MCL appear to take place in regions modulated during normal B-cell differentiation, however a few appear to be tumor specific.
Elias Campo also stated that studies performing genomic sequencing have confirmed relative high frequency of ATM and TP53 mutations, as well as discovering novel genes that are components of different pathways such as NOTCH, chromatin modification, or NFkB. TP53 mutations have been reported to be associated with a more aggressive evolution in both the classical and leukemic non-nodal subtypes of MCL.
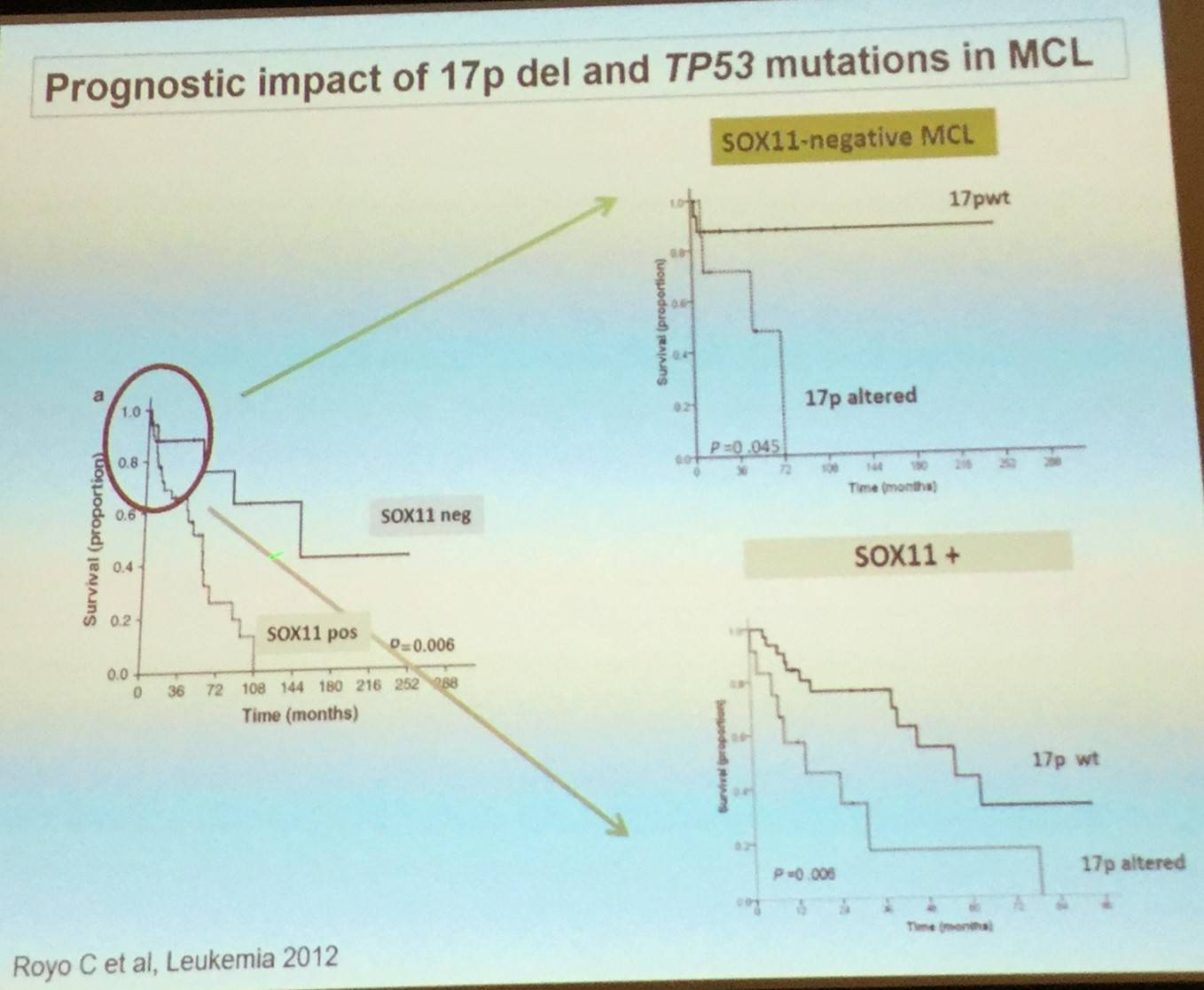
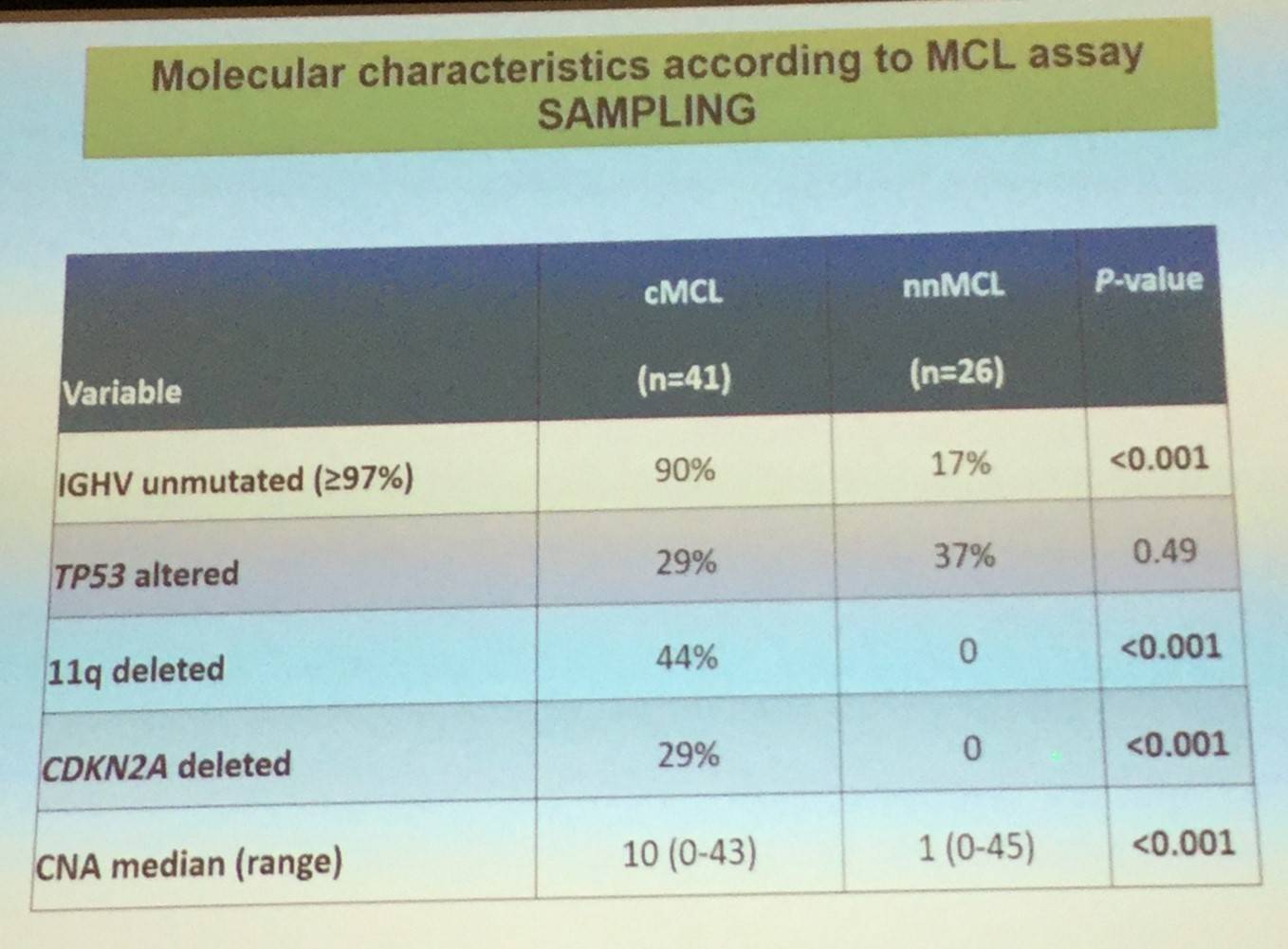
These findings increase and broaden our understanding of MCL pathogenesis and offer new insights into novel therapeutic interventions and monitoring of the disease.
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

