All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
EHA-SWG 2017 | Rare Lymphomas: Hairy Cell Leukemia
On March 10–12 2017, the EHA-SWG meeting on Rare Lymphomas took place in Barcelona, Spain, and was jointly chaired by Prof. Martin Dreyling, from Klinikum der Universität München, Germany, and Prof. Marie-José Kersten, from the Academic Medical Center, Amsterdam, The Netherlands.
On March 11th 2017, Tadeusz Robak from the Medical University of Lodz, Poland, gave a talk on Hairy Cell Leukemia (HCL) during the “MALT Lymphomas and HCL” scientific session.
The talk began by focusing on the epidemiology of HCL.
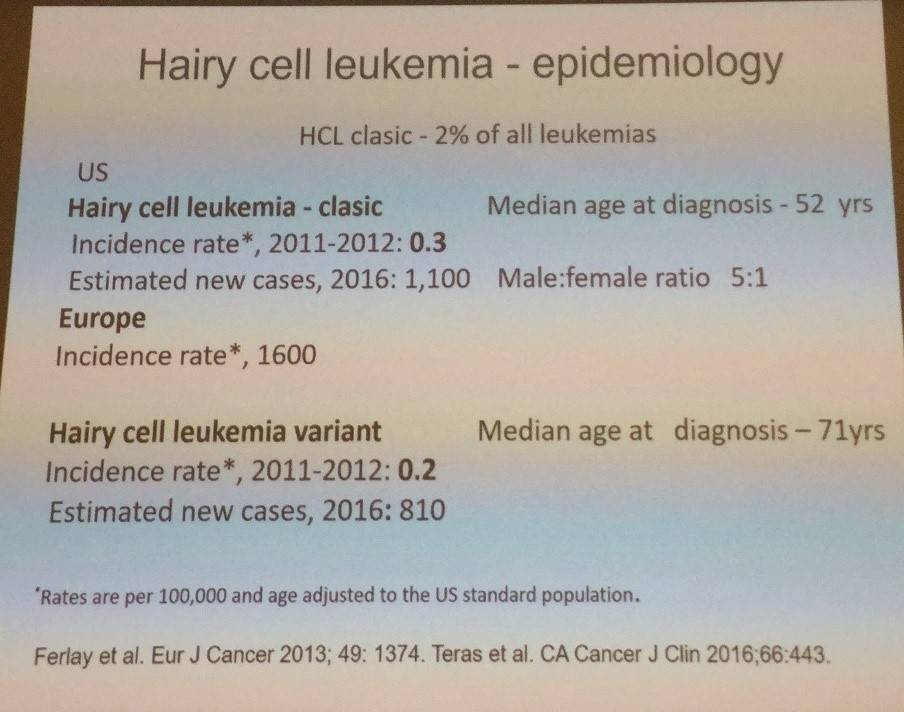
HCL is a rare type of CLL that stems from a mature B-lymphocyte. The disease is characterized by fatigue, infections, marked splenomegaly (in around 90%) and/or enlarged liver, progressive pancytopenia, and reactive marrow fibrosis. Peripheral lymphadenopathy is uncommon and leukemic cells are often rare.
Tadeusz Robak then talked through the diagnostic workup of classical HCL:
- Full blood cell counts, peripheral blood film morphology
- Bone marrow aspiration and trephine biopsy – H&E stain, reticulin stain, and immunohistochemistry for CD20, Annexin-1, DBA-44
- Immunophenotypic analysis by flow cytometry: CD19+, CD20+, CD11c+, CD25+, CD103+, CD123+
- BRAF V600E
- Tartrate-resistant acid phosphatase
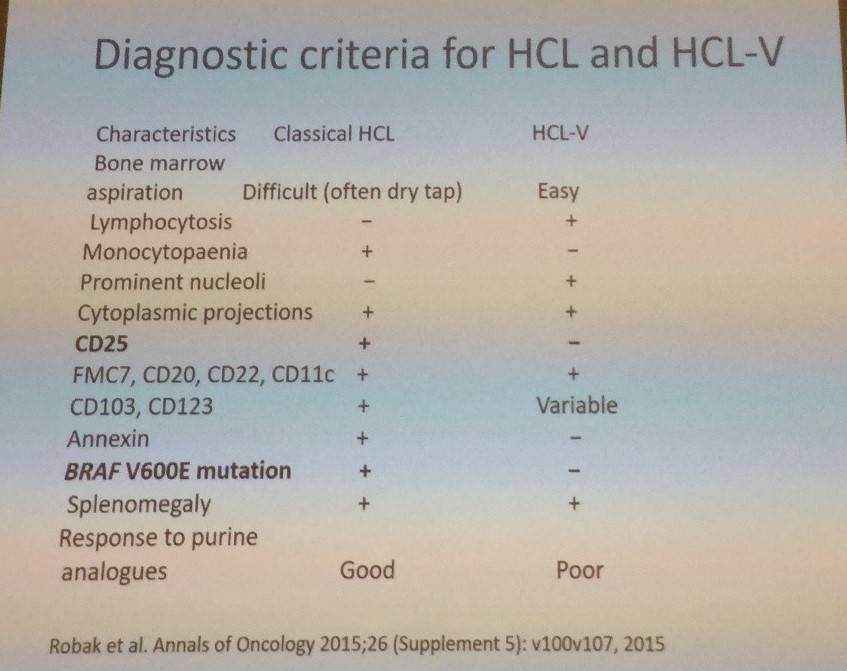
The talk then focused on the BRAF V600E mutation. In a report by Tiacci et al. (2011. N Engl J Med), Sanger sequencing detected mutated BRAF in 100% of 46 HCL patients. Variant-HCL is BRAF V600E negative. Morphologically similar diseases to classic HCL are splenic MZL and unclassifiable B-Cell Lymphomas, but these are BRAF V600E negative. Tadeusz Robak also talked through the differences in diagnostic criteria for classic and variant HCL:
Treatment is not indicated in asymptomatic classic HCL patients. Untreated patients should be closely monitored and a complete blood count with differential test should be carried out every 3–6 months. Indications for treatment are based on hematologic parameters (include ≥1 of the following):
- Hemoglobin <11g/dL
- Platelets <100,000/μl
- Neutrophils <1,000/μl
Clinical features or symptoms can also inform whether treatment is needed:
- Symptomatic organomegaly, progressive lymphocytosis or lymphadenopathy
- Unexplained weight loss (>10% within prior 6 months)
- Excessive fatigue (greater than NCI CTCAE grade 2)
Methods of evaluating response were also shared during the talk:
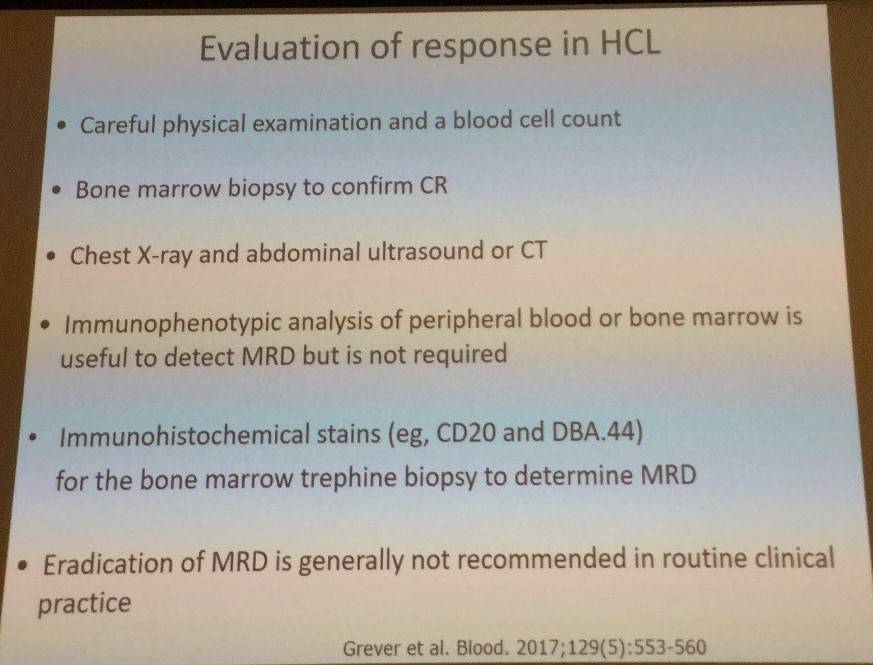
With cladribine, treatment response should be assessed after 4–6 months; with pentostatin, treatment response should be assessed after 8–9 courses.
Complete remission is defined by normalization of peripheral blood counts:
- Hemoglobin >11g/dL
- Platelets >100,000/μl
- Neutrophils >1,500/μl
In addition to this, the absence of morphologic evidence of HCL both in peripheral blood smear and bone marrow examination is required as well as regression of splenomegaly on physical examination.
Partial remission is defined by near normalization of the peripheral blood count (as in CR) and a minimum of 50% improvement in both the organomegaly and bone marrow biopsy infiltration with HCL.
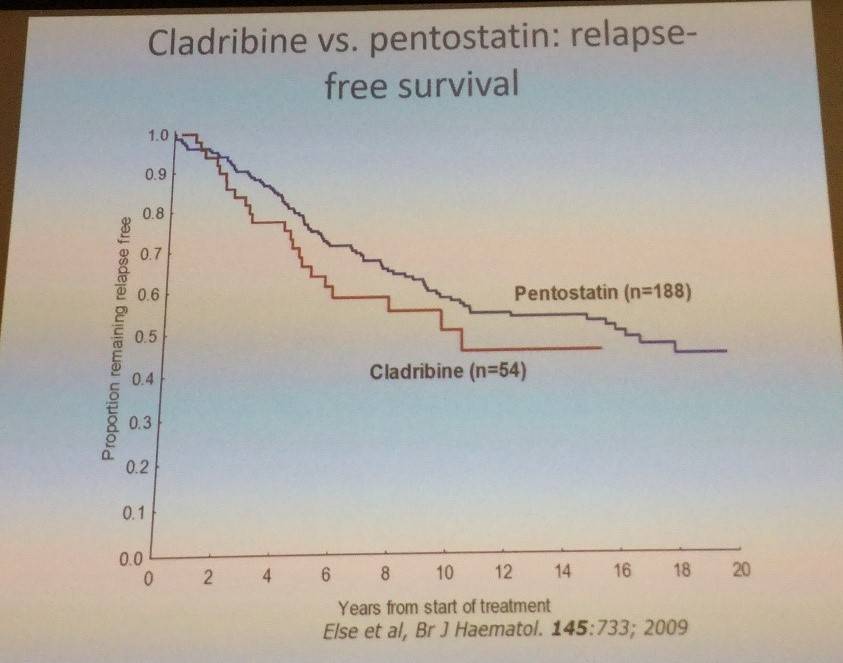
Tadeusz Robak then talked through first-line therapy using cladribine:

Robak et al. have reported findings with cladribine in the treatment of classic HCL in the Annals of Oncology in 2015.
- CR rate after single course = 85–91%
- CR and PR are similar regardless of weekly or daily administration, and when given as SC injection
- SC appears easier to administer than IV due to not requiring hospitalization
- Lower, compared to higher, total doses (0.5mg/kg vs 7mg/kg)) are associated with fewer cases of grade 3–4 toxicity (mainly neutropenic fevers and infections)
- Patients who achieve PR after the first course should receive a second course of cladribine (alone or in combination with rituximab) for at least 6 months after the end of the first course in order to achieve a CR
Treatment using pentostatin was also outlined: it is typically given at a dose of 4mg/m2 IV every second week until CR, plus 1–2 consolidating injections (in patients with normal creatinine clearance, >60mL/min). To confirm CR, a bone marrow biopsy should be performed.
The current indications for splenectomy in HCL were outlined:
- Patients with resistant massive symptomatic splenomegaly (>10cm below the costal margin) accompanied by low-level bone marrow infiltration
- HCL in pregnancy
- Patients refractory to nucleoside analogs and IFN-α
OR rates of 60–100% have been documented across eight major studies. Tadeusz Robak also stressed that systemic therapy should not be carried out earlier than 6 months after splenectomy.
The current indications for interferon-α in HCL were also discussed:
- HCL in pregnancy
- Patients presenting with severe neutropenia (neutrophils <0.2x109/L) to increase neutrophil count prior to nucleoside analog therapy
Following this, Tadeusz Robak then discussed supportive treatment options for HCL:
- Prophylactic co-trimoxazole and acyclovir for patients treated with nucleoside analogs with lymphopenia <1x109/L
- Myeloid growth factors need to be considered in patients with neutropenia and active infection (not routine adjunctive use)
- Patients may receive vaccinations that utilize killed viral agents (live virus vaccines should be avoided)
- Irradiated blood products should be transfused in patients treated with nucleoside analogs
- Screening for previous exposure to hepatitis before therapy recommended
The talk then focused on morphological and hematological relapse of HCL. Hematological relapse is the reappearance of cytopenia(s) below the thresholds defined for CR and PR. In morphological relapse, HCL reappears in the peripheral blood or bone marrow in the absence of hematological relapse. No treatment is required for morphological relapse. Things to take into account when deciding treatment for hematological relapse include reoccurrence of disease-related symptoms (e.g. splenomegaly) or progressive anemia, thrombocytopenia, or neutropenia.
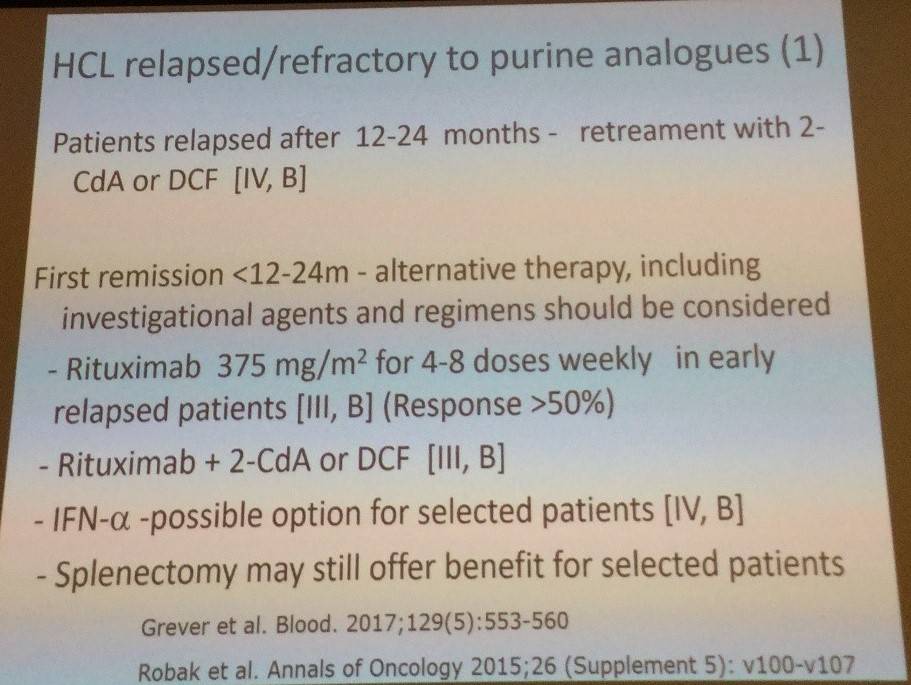
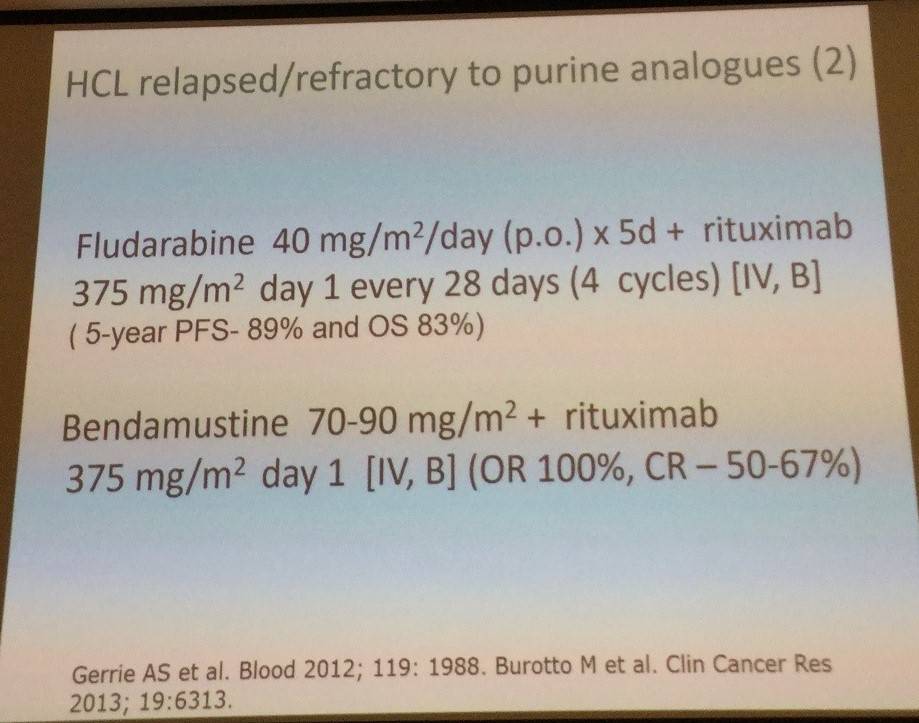
Tadeusz Robak then discussed new drugs for HCL treatment, the first being moxetumomab pasudotox (HA22, CAT-8015), an immunotoxin composed of variable heavy (VH) and light chains (VL) of the anti-CD22 mAb RFB4 linked by a disulfide bond, fused via a peptide linker to a 38-kDA fragment of Pseudomonas exotoxin. In a phase I trial (Kreitman et al. 2012. J Clin Oncol):
- All dose levels >5mcg/kg
- OR = 86% (42/49); CR = 46%
- Median PFS = not reached (exceeds 3 years)
- Most common AEs: hepatic transaminase elevation, hypoalbuminemia
- No dose limiting toxicities
- Majority of patients develop anti-drug antibodies
- Phase III monotherapy trial currently underway
The second new drug discussed was vemurafenib:
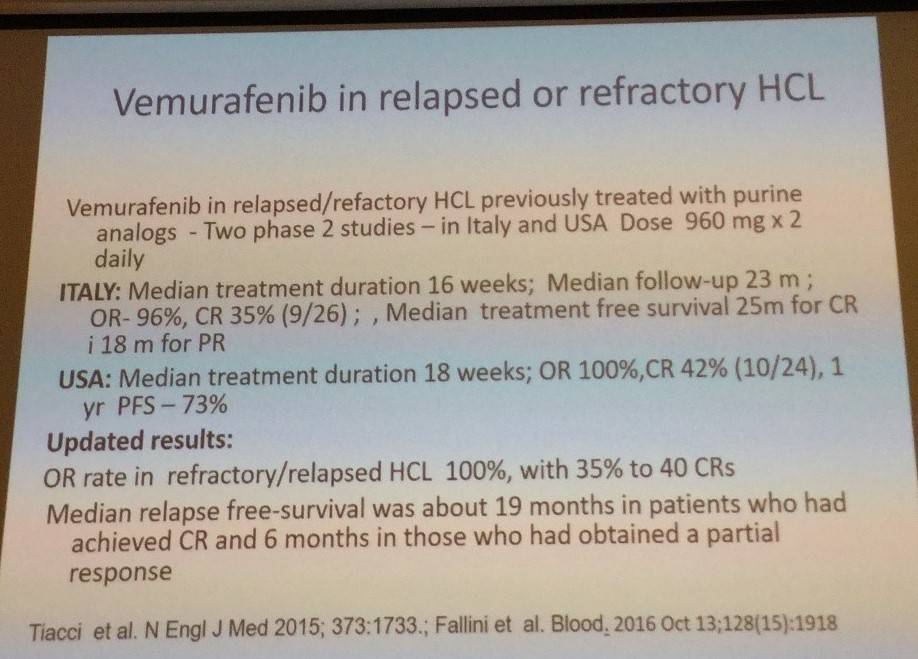
Low-dose vemurafenib has also been evaluated in refractory HCL (Dietrich et al. 2016. Blood):
- 21 R/R HCL patients; 240–1,920mg/d vemurafenib for median 90 days
- CR = 40%; rapid improvement of blood counts
- Median EFS = 17 months
- Vemurafenib can be given in neutropenic patients with active infection
- Retreatment in 6 patients with vemurafenib led to similar response patterns
- Median time to recovery:
- Platelets = 28 days (range, 10–105 days)
- Neutrophils = 43 days (range, 9–126 days)
- Hemoglobin = 55 days (range, 10–181 days)
- Low doses (2 x 240mg/day) are highly effective in refractory HCL
Tadeusz Robak then discussed ibrutinib for relapsed HCL:
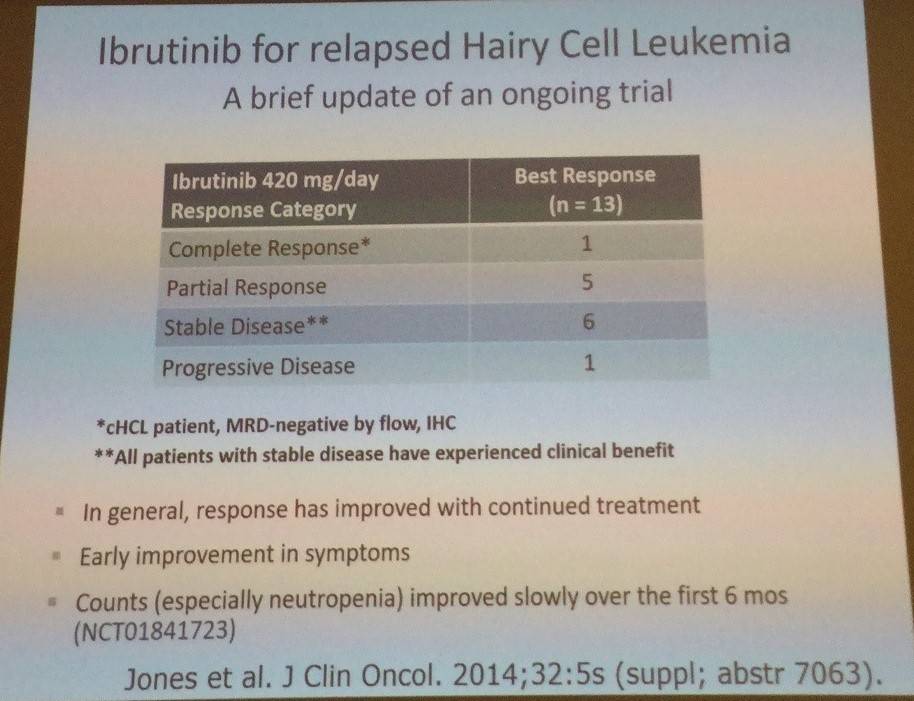
Following this, the role of allogeneic stem cell transplantation was also covered, with the main message being that it has a potential role in younger, heavily pretreated HCL patients who have had multiple relapses and are refractory to purine analogues and rituximab.
Tadeusz Robak finished the talk by outlining the ESMO clinical practice guidelines for diagnosis, treatment, and follow-up for classic and variant HCL:
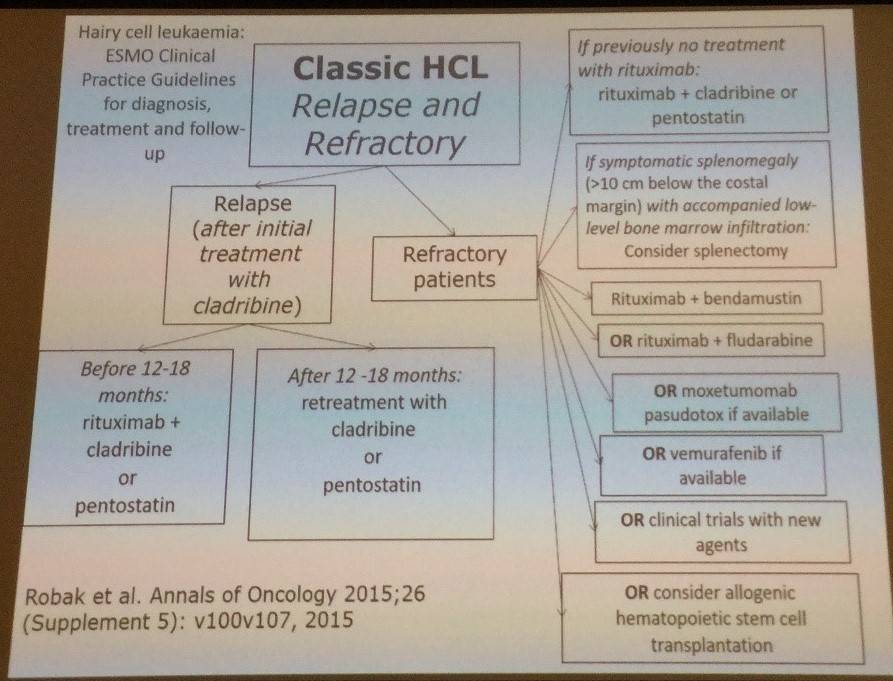
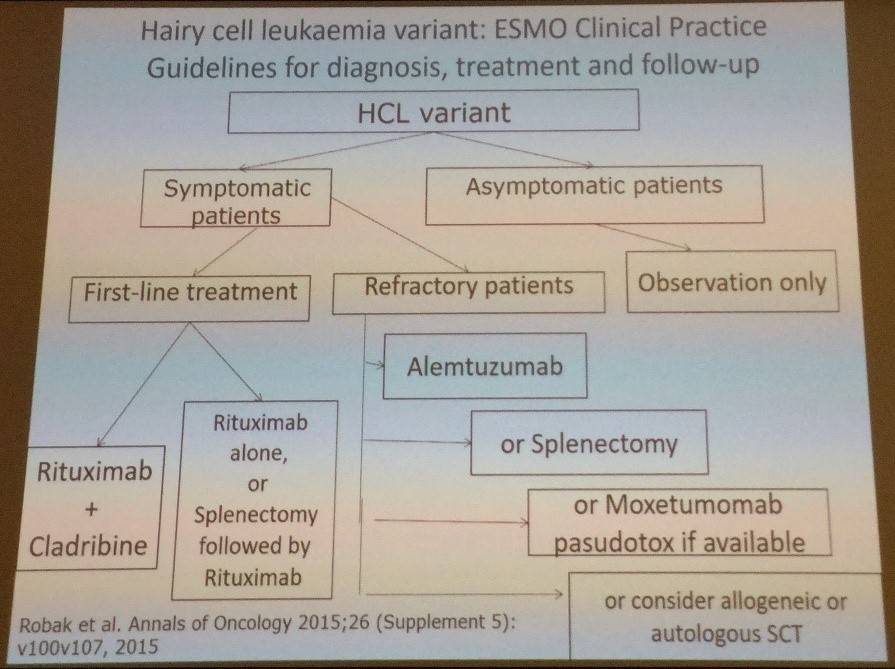
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

