All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
First-MIND trial: Safety of tafasitamab plus R-CHOP for newly diagnosed DLBCL
The combination of rituximab with cyclophosphamide, doxorubicin, prednisone, and vincristine (R‑CHOP) is the standard frontline treatment for newly diagnosed diffuse large B-cell lymphoma (DLBCL). Although R-CHOP has led to improved prognosis for many patients, around 15–20% of treatment-naïve patients have tumors with downregulated expression of CD20 and therefore have poorer responses to rituximab-containing regimens. Tafasitamab is an anti-CD19 Fc-modified monoclonal antibody therapy that enhances antibody-dependent cellular cytotoxicity and phagocytosis. Tafasitamab is currently approved by the U.S. Food and Drug Administration (FDA) in tandem with lenalidomide for treating relapsed/refractory DLBCL in patients not eligible for autologous stem cell transplant.
At the European Hematology Association (EHA)2021 Virtual Congress, David Belada presented a poster highlighting results from the First-MIND trial (NCT04134936), a phase 1b open-label trial investigating the safety and efficacy of tafasitamab plus R-CHOP or tafasitamab and lenalidomide plus R-CHOP for adult patients with newly diagnosed DLBCL. We summarize key results below.
Study design
The First-MIND trial enrolled 83 patients from 34 sites in Europe and the US. A total of 66 adult patients with newly diagnosed DLBCL were randomized 1:1 to the following treatment arms:
- Arm A: R-CHOP + tafasitamab (12 mg/kg intravenously).
- Arm B: R-CHOP + tafasitamab (12 mg/kg intravenously) + lenalidomide (25 mg orally).
- In addition, patients were given mandatory granulocyte stimulating factor prophylaxis, and for Arm B only, mandatory venous thromboembolism prophylaxis.
- Treatment consisted of a total of six 21-day treatment cycles, with dosing schedules for both treatment arms summarized in Figure 1.
Figure 1. Dosing schedule for 21-day treatment cycles*
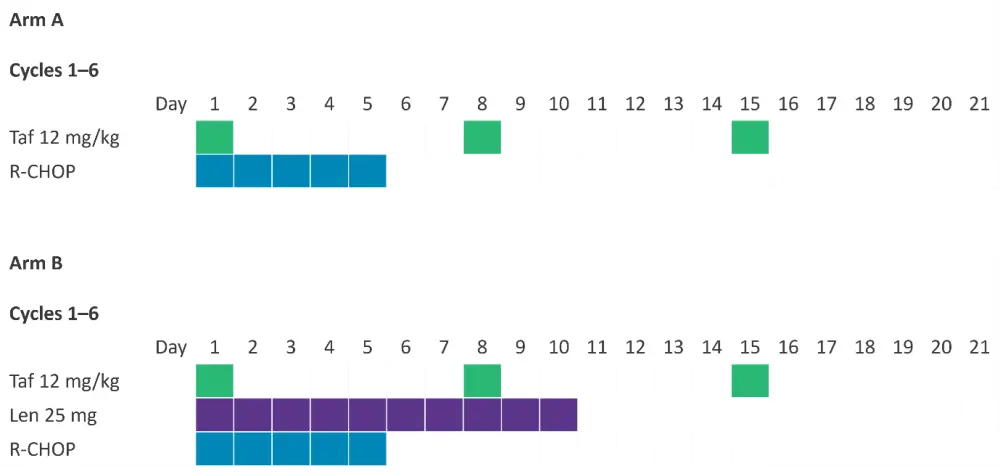
Len, lenalidomide; R-CHOP; rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; Taf, tafasitamab.
*Adapted from Belada, et al.1
Eligibility criteria
Eligibility criteria for this study included adults aged ≥18 years, treatment-naïve DLBCL, an International Prognostic Index of 2–5, and an Eastern Cooperative Oncology Group performance score of 0–2.
Exclusion criteria included patients with double- or triple-hit lymphoma, transformed non-Hodgkin lymphoma, evidence of composite lymphoma, history of radiation therapy to ≥25% of the bone marrow for other diseases, history of anthracycline therapy, known central nervous system involvement, or active hepatitis B/C infection.
Results
Patient characteristics are summarized in Table 1.
Table 1. Baseline patient characteristics*
|
DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; PS, performance status; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone |
||
|
Characteristic |
Arm A: R-CHOP + tafasitamab |
Arm B: R-CHOP + tafasitamab + lenalidomide |
|---|---|---|
|
Median age, years (range) |
66 (43–86) |
64 (20–79) |
|
Female, % |
54.5 |
60.6 |
|
Ann Arbor disease stage, % |
|
|
|
I |
6.1 |
3.0 |
|
II |
0 |
3.0 |
|
III |
21.2 |
21.2 |
|
IV |
69.7 |
72.7 |
|
Missing |
3 |
0 |
|
IPI risk score, % |
|
|
|
2 |
33.3 |
27.3 |
|
3 |
42.4 |
45.5 |
|
4 |
24.2 |
24.2 |
|
5 |
0 |
3 |
|
Bulky disease present, % |
42.4 |
45.5 |
|
ECOG PS, % |
|
|
|
0 |
60.6 |
33.3 |
|
1 |
30.3 |
57.6 |
|
2 |
9.1 |
9.1 |
|
Reference diagnosis DLBCL (central pathology review), % |
|
|
|
DLBCL |
90.9 |
90.9 |
|
Other |
3.0 |
9.1 |
|
Missing |
6.1 |
0 |
Primary endpoint
Over a follow-up of 18 months, the most frequent TEAEs were blood and lymphatic disorders. The relative platelet and neutrophil counts remained consistent between both treatment arms across all six cycles of treatment, however the incidences of Grade ≥3 cytopenia and thrombocytopenia were higher for patients treated with tafasitamab plus lenalidomide. In total, six patients received platelet transfusion; three patients in each arm. Grade ≥3 TEAEs are summarized in Table 2.
Table 2. Grade ≥3 hematologic and non-hematologic TEAEs*
|
R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; TEAE, treatment-emergent adverse event. |
||
|
Grade ≥3 TEAE, % |
Arm A: R-CHOP + tafasitamab |
Arm B: R-CHOP + tafasitamab + lenalidomide |
|---|---|---|
|
Hematologic |
|
|
|
Neutropenia |
57.6 |
84.8 |
|
Anaemia |
21.2 |
27.3 |
|
Thrombocytopenia |
9.1 |
33.3 |
|
Leukopenia |
18.2 |
27.3 |
|
Febrile cytopenia |
18.2 |
18.2 |
|
Lymphopenia |
12.1 |
21.2 |
|
Non-hematologic |
|
|
|
Diarrhea |
3.0 |
6.1 |
|
Hypokalaemia |
9.1 |
6.1 |
|
Peripheral neuropathy |
0 |
3.0 |
|
Vomiting |
3.0 |
0 |
|
Constipation |
0 |
3.0 |
|
Asthenia |
3.0 |
0 |
|
Fatigue |
3.0 |
0 |
|
Stomatitis |
3.0 |
0 |
|
Hypotension |
3.0 |
9.1 |
|
Infusion-related reaction |
0 |
3.0 |
|
Abdominal pain |
0 |
3.0 |
- Serious TEAEs were reported in 42.4% of patients in Arm A and 51.5% of patients in Arm B.
- Three deaths were reported, none of which were attributable to tafasitamab or lenalidomide treatment.
- The median average relative dose intensity of R-CHOP was unchanged in either treatment arm, remaining at 100% throughout all six cycles of treatment.
- A total of 21 patients experienced treatment cycle delay overall, and the percentage of patients reporting delays in each cycle are summarized in Table 3.
Table 3. Number of patients with a treatment cycle delay within each cycle*
|
R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. |
||
|
Cycle number, % |
Arm A: R-CHOP + tafasitamab |
Arm B: R-CHOP + tafasitamab + lenalidomide |
|---|---|---|
|
Cycle 2 |
12.1 |
6.1 |
|
Cycle 3 |
3.0 |
0.0 |
|
Cycle 4 |
12.1 |
6.1 |
|
Cycle 5 |
3.0 |
9.1 |
|
Cycle 6 |
3.0 |
9.1 |
Secondary endpoints
In total, 60 patients were assessed for overall response at the end of treatment. The ORR for both arms combined was 83.3% (95% confidence interval, 71.5–91.7) and the complete remission rate was 75.0% (95% confidence interval, 62.1‒85.3).
Conclusion
Overall, the First-MIND trial demonstrated the tolerability of tafasitamab and lenalidomide in addition to R-CHOP for treatment-naïve DLBCL. No unexpected toxicity was observed, and while Grade ≥3 neutropenia frequency was higher with tafasitamab plus lenalidomide (84.8%) versus tafasitamab alone (57.6%), the incidence of febrile neutropenia was equal (18.2%). Additionally, the relative dosage intensity of R-CHOP was unaffected with the addition of both therapies. Efficacy analysis provided promising evidence for response with this triplet combination, with a combined ORR of 83.4% and complete remission rate of 75.0%.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

