All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Frontline ibrutinib for younger patients with MCL: Results from the phase III TRIANGLE trial
Mantle cell lymphoma (MCL) is an uncommon form of non-Hodgkin lymphoma, occurring 5–10% of patients with non-Hodgkin lymphoma globally. The treatment landscape for MCL is rapidly evolving. Currently, high-dose cytarabine-containing immunochemotherapy followed by autologous stem cell transplantation (ASCT) and rituximab maintenance therapy represents the standard of care (SOC) for younger patients with MCL. In recent years, Bruton's tyrosine kinase (BTK) inhibitors have shown promise as a more effective treatment protocol than SOC treatments, with ibrutinib demonstrating promising efficacy in relapsed and previously untreated older patients with MCL.
In younger patients with advanced MCL, it is unclear whether incorporation of ibrutinib into frontline therapy could improve outcomes. To address this, the European MCL Network is conducting the phase III, randomized, open-label TRIANGLE trial (NCT02858258) evaluating the addition of ibrutinib to standard treatment in comparison to the previous standard treatment and an ibrutinib regimen without ASCT.
Here, we report a summary of results from the ongoing phase III TRIANGLE trial of ibrutinib in younger patients with MCL presented by Dreyling at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition in December 2022, as part of the plenary scientific session.
Study design
The trial design is shown in Figure 1.
Figure 1. Trial design*†
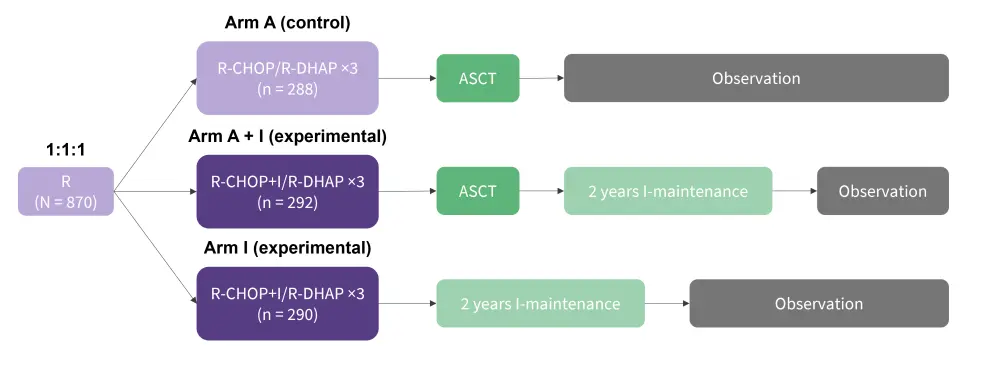
ASCT, autologous stem cell transplantation; l, ibrutinib; R, rituximab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-DHAP, rituximab, dexamethasone, cytarabine, cisplatin.
*Adapted from Dreyling, et al.1
†Rituximab maintenance was added in all three arms and was started (+/− ibrutinib) in 58%/57%/54% of A/A+I/I randomized patients, respectively.
TRIANGLE is an ongoing phase III, randomized, open-label, three-arm trial of ibrutinib to compare the effectiveness and safety of adding ibrutinib to standard treatment (arm A + I) vs previous standard treatment (arm A) and an ibrutinib containing treatment without ASCT (arm I) (Figure 1).
Between July 2016 and December 2020, 870 patients were selected for enrolment and randomized 1:1:1 to the three trial arms. Eligible patients were aged ≤65 years, had advanced previously untreated Stage II–IV MCL, an Eastern Cooperative Oncology Group Performance Score of 0–2, and were suitable for high-dose cytarabine and ASCT therapy.
The primary outcome was failure-free survival (FFS), with stable disease at the end of induction, progression, and death constituting qualifying events. Key secondary outcomes included response rates, progression-free survival, overall survival, and safety.
Results
Figure 2. Efficacy outcomes*
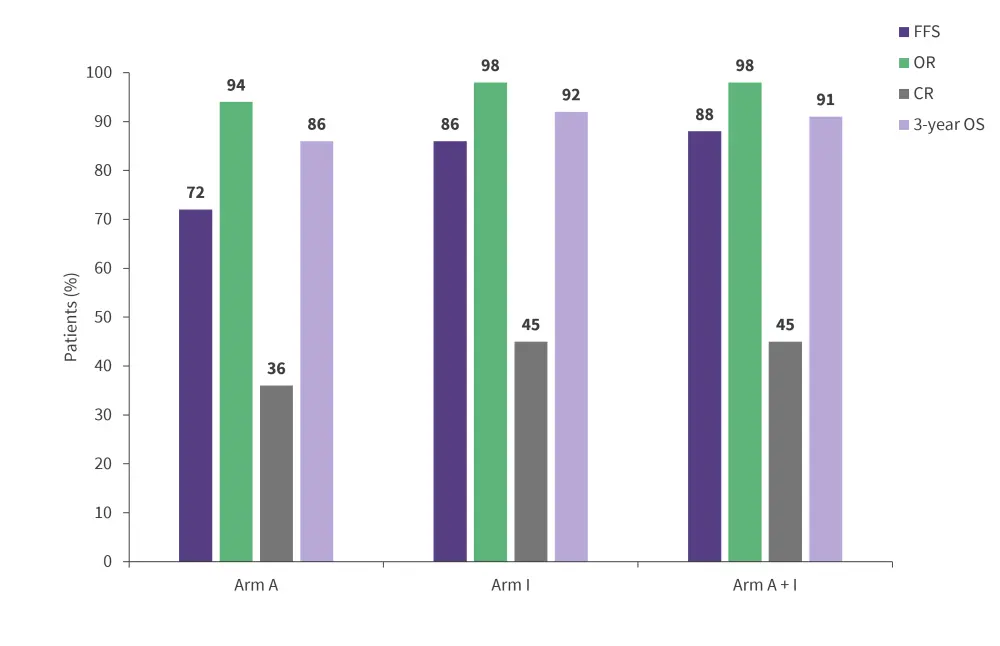
CR, complete response; FFS, failure-free survival; OR, overall response; OS, overall survival.
*Adapted from Dreyling, et al.1
Efficacy1
- OR and CR rates were 94% and 36% in arm A vs 98% and 45% in the A+I/I arms combined, respectively.
- After a median follow-up of 31 months, 3-year FFS was 72% vs 86% in arm A vs arm I; this showed that previous standard treatment was not superior to ibrutinib containing treatment without ASCT. Arm A + I showed improved FFS over arm A, with a 3-year FFS of 88% vs 72%, respectively (Figure 2).
- Three-year overall survival for arms A, A + l, and l was 86%, 91%, and 92%, respectively.
- Subgroup analyses by the intention to apply rituximab maintenance did not affect the study results.
- Statistical monitoring for the FFS comparison of arm A + I vs arm I is ongoing.
Safety1
- No significant differences in the occurrence of Grade 3–5 adverse events (AEs) were detected between induction with R-CHOP/R-DHAP vs ibrutinib-R-CHOP/R-DHAP patient groups.
- The two ASCT-containing arms (arm A and arm A + l) did not show significant differences in the occurrence of Grade 3–5 AEs.
- During the maintenance phase, patients in the A + I arm experienced more AEs than those in arms A and l, with
- 44%, 17%, and 23% experiencing neutropenia;
- 4%, 2%, and 2% experiencing leukopenia;
- 6%, 3%, and 3% experiencing febrile neutropenia;
- 25%, 13%, and 19% experiencing infections and infestations; and
- 3%, 1%, and 4% experiencing cardiac disorders, respectively.
Conclusion
These results show that addition of ibrutinib to ASCT leads to improved FFS outcomes. In addition, neither ASCT-containing arm (arm A and arm A + l) had significantly better outcomes compared with the ibrutinib alone arm (arm l); however, therapy with ibrutinib alone had a more favorable safety profile. Longer-term follow-up results will indicate if these results remain consistent in younger patients with MCL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

