All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
GLOW study: Impact of MRD on PFS in older and frail patients with CLL receiving fixed-duration ibrutinib + venetoclax vs chlorambucil + obinutuzumab
Do you know... What were the progression-free survival (PFS) rates 12 months after the end of treatment with ibrutinib + venetoclax, for patients with undetectable minimal residual disease (uMRD) and detectable minimal residual disease (dMRD)?
The phase III GLOW trial (NCT03462719), which has been previously reported on the Lymphoma Hub, evaluated the efficacy and safety of fixed-duration ibrutinib + venetoclax vs chlorambucil + obinutuzumab in patients with previously untreated chronic lymphocytic leukemia (CLL) who were older and/or had comorbidities. The results showed superior progression-free survival (PFS) and significantly higher undetectable minimal residual disease (uMRD < 10−4) rates in bone marrow for ibrutinib + venetoclax vs chlorambucil + obinutuzumab.
While minimal residual disease (MRD) can be associated with PFS after fixed-duration treatments such as chemotherapy, this association has not yet been evaluated for ibrutinib + venetoclax. Munir T et al.1 analyzed results from the GLOW trial to evaluate any potential association between MRD and PFS outcomes for ibrutinib + venetoclax compared with chlorambucil + obinutuzumab. The results are summarized in this article.
Study design1
This is a retrospective analysis of the phase III GLOW trial, in which 211 patients were randomly assigned 1:1 to receive ibrutinib + venetoclax or chlorambucil + obinutuzumab. Patients were stratified by immunoglobulin heavy-chain variable region (IGHV) mutational status and presence of 11q deletion. Patients with del17p or known TP53 mutations were excluded.
Methods
uMRD was assessed by next-generation sequencing at <1 CLL cell per 10,000 (<10-4) and <1 CLL cell per 100,000 (<10-5) leukocytes. Detectable MRD (dMRD) was defined as having ≥1 CLL cell per 10,000 leukocytes (≥10-4). PFS was analyzed by MRD status at 3 months after treatment (EOT+3).
Results1
A total of 106 patients received ibrutinib + venetoclax and 105 received chlorambucil + obinutuzumab, with well-balanced baseline characteristics between the treatment groups (Table 1).
Table 1. Baseline characteristics*
|
CIRS, Cumulative Illness Rating Scale; CrCl, creatinine clearance; del11q, 11q deletion; ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy-chain variable region; LDH, lactate dehydrogenase. *Adapted from Munir, et al. |
||
|
Characteristic, % (unless otherwise specified) |
Ibrutinib + venetoclax |
Chlorambucil + obinutuzumab (N = 105) |
|---|---|---|
|
Median age (range), years |
71.0 (47–93) |
71.0 (57–88) |
|
Male |
55.7 |
60.0 |
|
Female |
44.3 |
40.0 |
|
ECOG PS 1–2 |
67.0 |
62.9 |
|
Median CIRS score (range) |
9 (1–20) |
8 (0–22) |
|
>6† |
69.8 |
58.1 |
|
Median CrCl,‡ mL/min (range) |
66.5 (34.0–168.1) |
63.2 (32.3–180.9) |
|
Rai stage III-IV |
57.3 |
52.5 |
|
Bulky disease ≥5 cm |
39.0 |
36.2 |
|
Elevated LDH† |
33.0 |
48.6 |
|
IGHV status |
||
|
Mutated |
25.5 |
25.7 |
|
Unmutated |
51.9 |
51.4 |
|
Unknown |
22.6 |
22.9 |
|
del11q |
18.9 |
17.1 |
|
TP53 mutation§ |
6.6 |
1.9 |
Efficacy
- PFS remained superior for ibrutinib + venetoclax versus chlorambucil + obinutuzumab (hazard ratio, 0.212; 95% CI, 0.129–0.349; p < 0.001).
- The estimated 30-month response rate was 86.7% in the ibrutinib + venetoclax arm vs 35.5% in the chlorambucil + obinutuzumab arm.
- Time to next treatment was prolonged for patients receiving ibrutinib + venetoclax vs chlorambucil + obinutuzumab.
- The risk of needing second-line therapy was reduced by 85.3% with first-line ibrutinib + venetoclax vs chlorambucil + obinutuzumab (hazard ratio, 0.147; 95% CI, 0.062–0.350; p < 0.0001).
MRD kinetics
MRD at EOT+3
A higher percentage of patients receiving ibrutinib + venetoclax compared with chlorambucil + obinutuzumab had uMRD (< 10-4) at EOT+3 in both bone marrow and peripheral blood analysis, as shown in Figure 1.
Figure 1. Percentage of patients with uMRD*
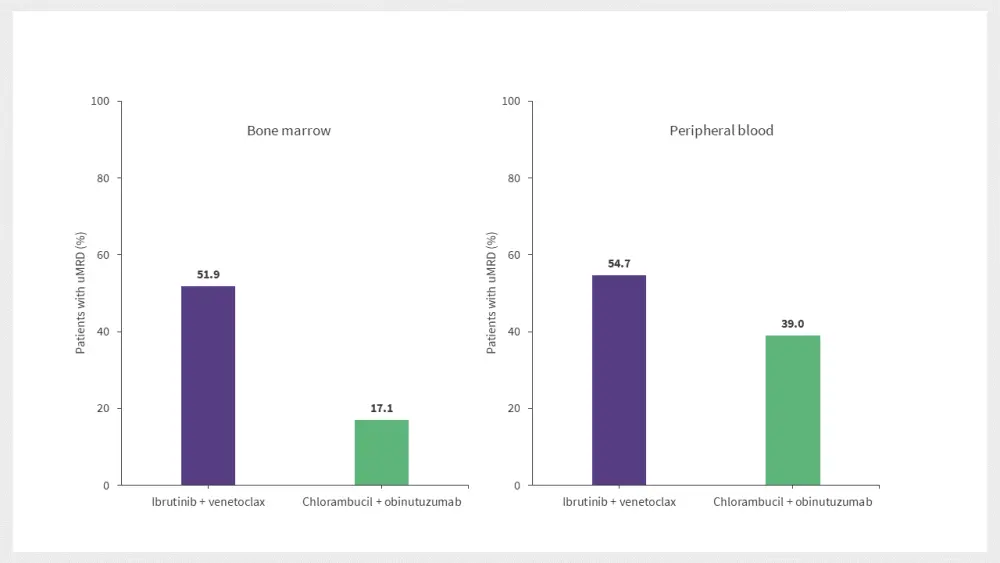
uMRD, undetectable minimal residual disease.
*Adapted from Munir et al.
Sustained MRD at EOT+12
At EOT+12, uMRD < 10-5 was higher for ibrutinib + venetoxlox vs chlorambucil + obinutuzumab, at 36.8% vs 6.7%, respectively. The uMRD rates were also better sustained during the first year of post-treatment for ibrutinib + venetoclax, as 80.4% of patients maintained uMRD < 10-5 compared with 26.3% in the chlorambucil + obinutuzumab arm.
Patients with dMRD ≥ 10-4 at EOT+3 in the ibrutinib + venetoclax arm were less likely to have increasing levels of MRD and/or progress clinically compared with patients in the chlorambucil + obinutuzumab arm.
PFS by MRD status, IGHV status, and tumor response
MRD status
The PFS rate was maintained irrespective of MRD status at EOT+3 in the ibrutinib + venetoclax arm, but not in the chlorambucil + obinutuzumab arm, as shown in Figure 2.
Figure 2. Impact of MRD status on the estimated PFS at EOT+12*
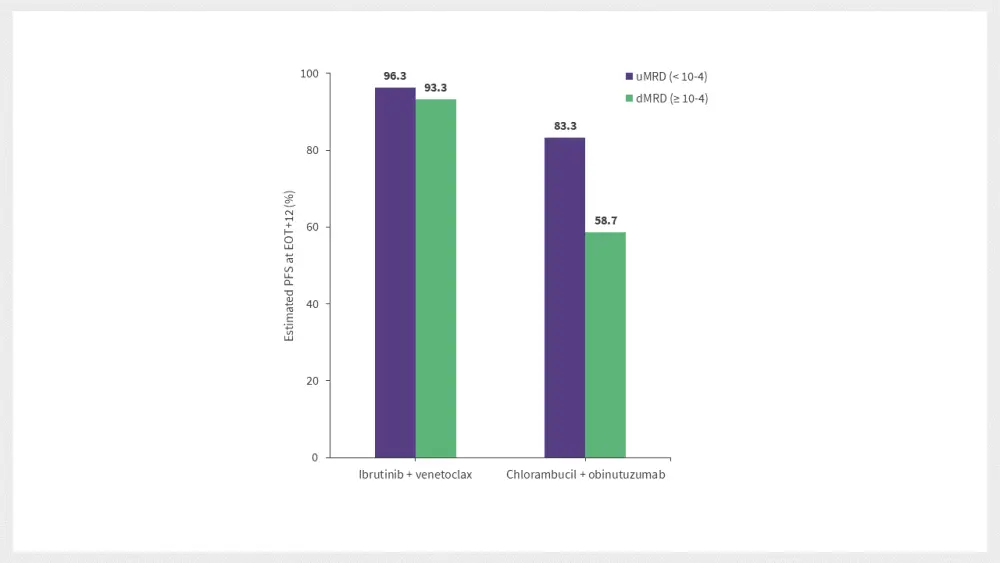
EOT+12, 12 months after end of treatment; dMRD, detectable minimal residual disease; uMRD, undetectable minimal residual disease.
*Data from Munir et al.
IGHV status
The PFS rates at EOT+12 also remained high in patients with unmutated immunoglobulin heavy-chain variable region (uIGHV) receiving ibrutinib + venetoclax, independent of MRD status in bone marrow (BM). Whereas PFS rates were lower and dependent on MRD status in the chlorambucil + obinutuzumab arm.
Tumor response
The 30-month PFS rates were similar regardless of whether the best tumor response was a complete response or partial response in the ibrutinib + venetoclax arm. In comparison, the 30-month PFS rate was lower in patients with a partial response vs complete response in the chlorambucil + obinutuzumab arm.
Safety
One patient in the chlorambucil + obinutuzumab arm was diagnosed with new serious treatment-emergent adverse events. Otherwise, no major changes in safety were noted at a median follow-up of 34.1 months compared with the primary analysis (27.7 months). The number of patients with a second primary malignancy during the study increased from 7.5% to 9.4% in the ibrutinib + venetoclax arm, and from 9.5% to 11.4% in the chlorambucil + obinutuzumab arm.
Conclusion
No association between MRD and PFS was found in this analysis by Munir et al, with PFS rates remaining high 12 months after the end of treatment with ibrutinib + venetoclax irrespective of MRD status. The results from this study also conclude that older and frail patients with CLL who receive ibrutinib + venetoclax are less likely to progress clinically during the first year of post-treatment regardless of their MRD and IGHV status, compared with chlorambucil + obinutuzumab.
The lack of association between MRD status and PFS outcomes at 12 months after treatment is a novel finding and requires further research to confirm these findings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

