All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Ibrutinib–rituximab combinations for frontline use in MCL
Do you know... IMCL-2015 is a phase II study evaluating ibrutinib plus rituximab for the frontline treatment of patients with indolent MCL. Which patient group demonstrated the highest overall response (CR + PR) following 12 cycles of therapy?
The treatment landscape for patients with mantle cell lymphoma (MCL) has progressed significantly in recent years with the emergence of targeted agents.1,2 There are a number of studies underway evaluating novel treatment combinations in both the frontline and relapsed/refractory setting, working towards a personalized treatment approach.1
Ibrutinib and rituximab are among the targeted agents used to treat MCL and are being investigated as a frontline combination, with the hope to limit patient exposure to chemotherapy.2 The Lymphoma Hub is happy to provide a summary of the data from two phase II studies investigating ibrutinib–rituximab combination regimens for the treatment of patients with MCL.
IMCL-20151
Study design
Patients with indolent presentations of MCL often demonstrate favorable survival, without the need for intensive chemotherapy. Personalized, chemotherapy-free regimens may be of particular benefit in the frontline indolent MCL setting.
The single-arm IMCL-2015 study (NCT02682641) enrolled patients aged ≥18 years with treatment-naïve, asymptomatic, indolent MCL (confirmed using World Health Organization diagnostic criteria) from across 12 centers. Patients had an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1, and those with blastoid presentation and/or Ki-67 >30% were ineligible for enrolment. The study evaluated frontline rituximab plus ibrutinib and employed a measurable residual disease (MRD)-directed approach to limit treatment duration.
Patients received treatment in 28-day cycles as follows:
- Rituximab (weekly intravenous [IV] dose of 375 mg/m2)
- Cycle 1: Days 1, 8, 15, and 22
- Cycles 3, 5, 7, and 9: Day 1
- Ibrutinib: 560 mg/day orally for 2 years if sustained MRD negativity is achieved, or until progression or intolerable toxicity.
Primary endpoint: Complete response (CR) in the intention-to-treat population following 12 cycles of treatment
Secondary endpoints: Overall response rate (ORR), rate of undetectable MRD (uMRD), progression-free survival (PFS), duration of response, overall survival (OS), safety, and tolerability
Results
- A total of 50 patients were enrolled (Figure 1, Table 1)
Figure 1. IMCL-2015 study profile*
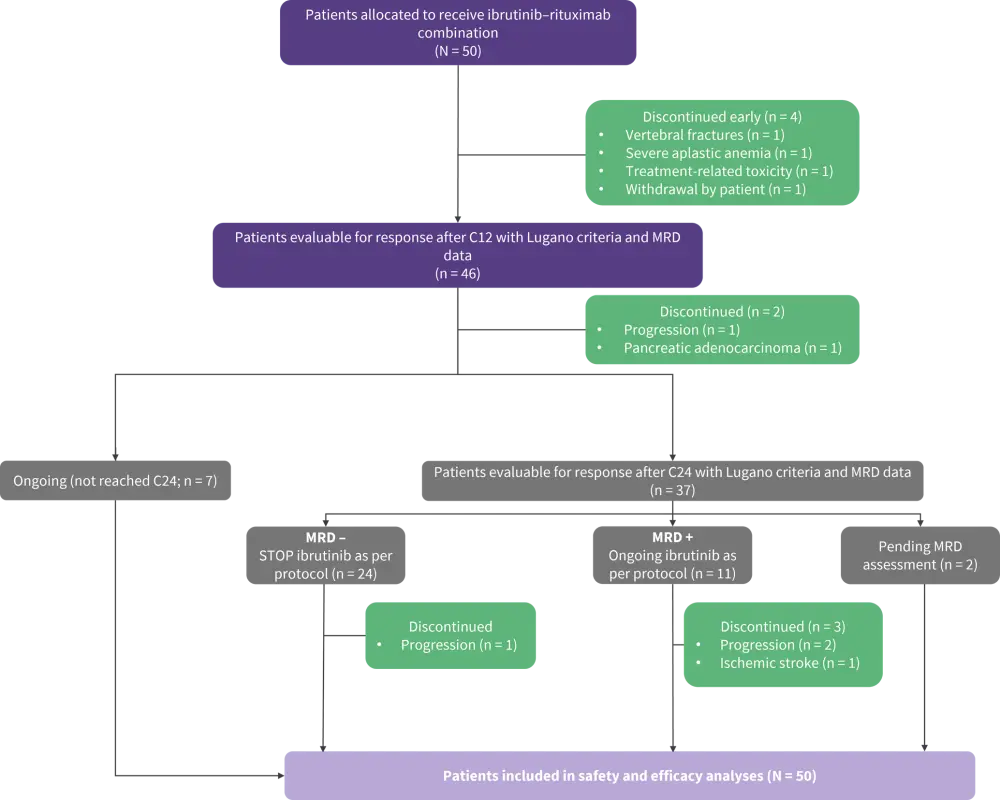
C12, Cycle 12; C24, Cycle 24; MRD, minimal residual disease.
*Adapted from Giné, et al.1
Table 1. Baseline characteristics of patients enrolled in the IMCL-2015 study*
|
BM, bone marrow; cMCL, conventional MCL molecular subtype; ECOG, Eastern Cooperative Oncology Group; MCL, mantle cell lymphoma; MIPI, Mantle Cell Lymphoma International Prognostic Index; nnMCL, non-nodal MCL molecular subtype; PB, peripheral blood. |
|
|
Characteristic (% unless stated otherwise) |
Patients |
|---|---|
|
Median age, years (range) |
65 (40–85) |
|
Sex |
|
|
Male |
66 |
|
Female |
44 |
|
BM involvement |
88 |
|
PB involvement by flow cytometry† |
90 |
|
MIPI risk |
|
|
Low |
24 |
|
Intermediate |
38 |
|
High |
38 |
|
TP53 alterations, n (%) |
6/41 (15) |
|
Del 17p/LOH, n |
5 |
|
TP53 mutation, n |
6 |
|
L-MCL-16 |
|
|
nnMCL |
55 |
|
cMCL |
45 |
Efficacy
- Following 12 cycles of the ibrutinib–rituximab regimen
- ORR across all patients was 84%, with a CR of 80% (Figure 2). uMRD in peripheral blood (PB) and bone marrow (BM) was achieved by 93% and 70% of patients with a complete response, respectively.
- uMRD in PB and BM was achieved by 87% and 65% of 46 evaluable patients, respectively.
Figure 2. Patient outcomes following 12 cycles of ibrutinib + rituximab by molecular subtype and TP53 mutational status*
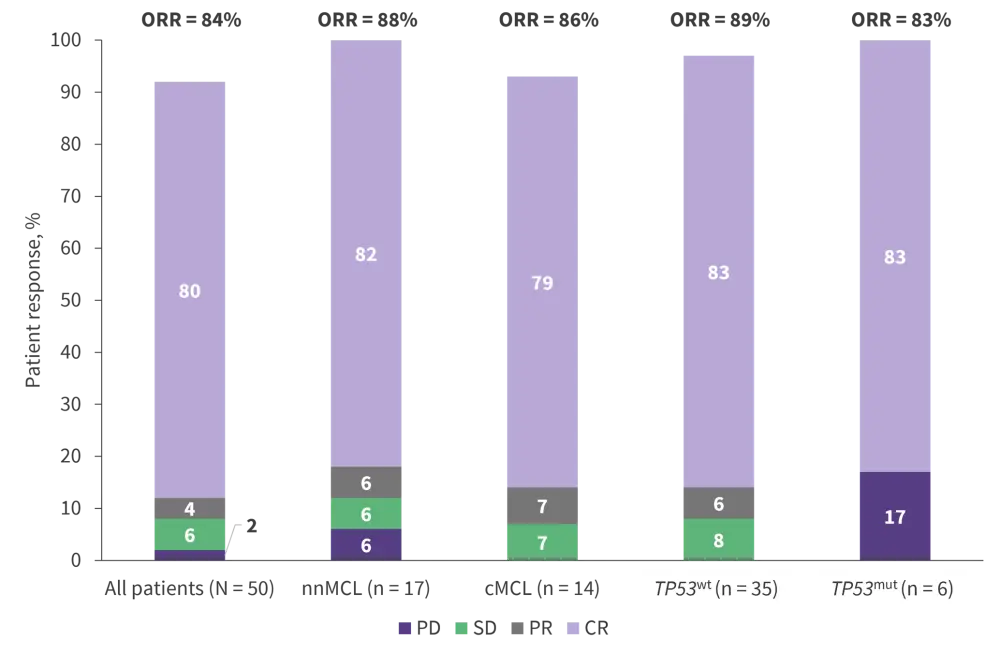
cMCL, conventional MCL molecular subtype; CR, complete response; mut, mutation; nnMCL, non-nodal MCL; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; WT, wildtype.
*Data from Giné, et al.1
- Sustained uMRD was achieved in 69% of patients who reached treatment Cycle 24 (Figure 1), and ibrutinib was discontinued. uMRD persisted in 19 patients at the latest data collection.
- The estimated 36-month PFS was 93% (95% confidence interval [CI], 86–100).
- EFS and OS at 36 months were 76% (95% CI, 64–89) and 92% (95% CI, 84–100), respectively, with six patients dying on study to date (MCL progression, n = 4; pancreatic adenocarcinoma, n = 1; SARS-CoV-2 infection, n = 1).
- PFS and OS were significantly influenced by MIPI and TP53 mutational status.
- Patients with TP53-mutated MCL had significantly lower median PFS compared with TP53wt cases (38.5 months vs not reached, respectively; p = 0.0001) and OS (38.5 months vs not reached, respectively; p = 0.0002).
Safety
- The most commonly observed treatment-related adverse events are shown in Table 2.
- Ibrutinib discontinuation due to AEs was reported in six patients.
Table 2. Treatment-related adverse events observed in ≥20% of patients in the IMCL-2015 study*
|
*Data from Giné, et al.1 |
|||
|
AE, % |
All grades |
Grade 3 |
Grade 4 |
|---|---|---|---|
|
Hematologic |
|
|
|
|
Thrombocytopenia |
14 |
0 |
2 |
|
Neutropenia |
36 |
16 |
6 |
|
Nonhematologic |
|
|
|
|
Diarrhea |
38 |
2 |
0 |
|
Fatigue |
32 |
2 |
0 |
|
Upper respiratory infection |
24 |
0 |
0 |
|
Nausea |
22 |
0 |
0 |
|
Hypertension |
20 |
2 |
0 |
IMCL-2015 study conclusions
In patients with indolent MCL, frontline ibrutinib plus rituximab demonstrated encouraging CR and uMRD rates, meeting the study’s primary endpoint. The presence of TP53 mutations was associated with poor outcomes. The trial utilized an MRD-guided treatment approach to identify avoidable treatment cycles and further optimize personalized care in the setting.
WINDOW-12
Study design
Although induction with intensive chemotherapy can induce favorable and durable responses in patients with MCL, AEs and the risk of second cancers remain significant drawbacks. WINDOW-1 (NCT02427620) is a single-arm, single-center study evaluating the safety and efficacy of chemotherapy-free induction with ibrutinib–rituximab (part A) followed by shortened consolidation with alternating R-HCVAD (rituximab plus hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and methotrexate–cytarabine (part B; Figure 3).
WINDOW-1 enrolled patients aged ≤65 years with treatment-naïve MCL, an ECOG PS of ≤2, serum bilirubin <1.5 mg/dL, and creatinine clearance ≥30 mL/min.
Primary endpoint: ORR following part A
Secondary endpoints: PFS, OS, CR, duration of response, and toxicities following part A and part B
Figure 3. WINDOW-1 treatment schema*
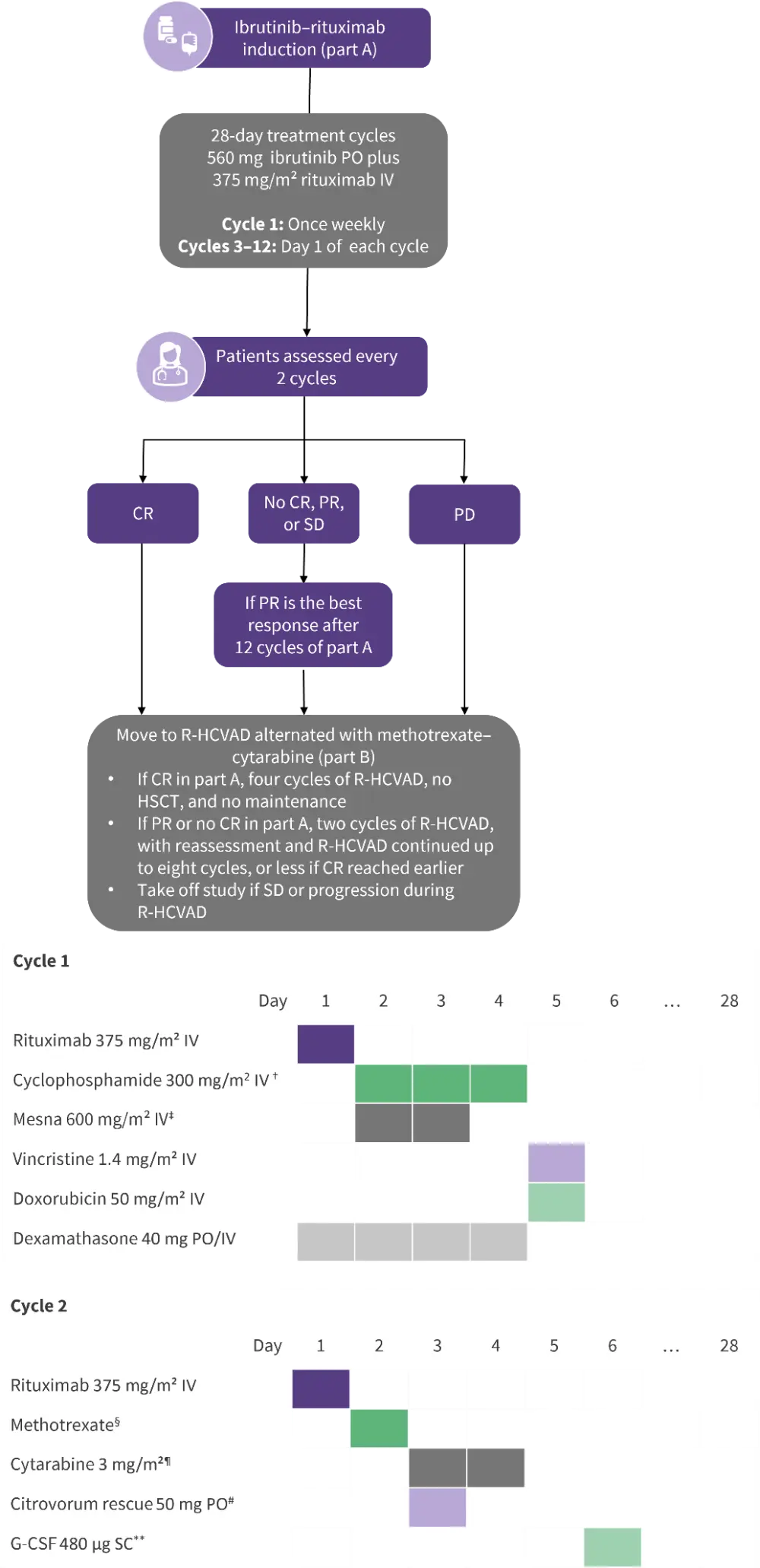
CR, complete response; HSCT, hematopoietic stem cell transplantation; IV, intravenous; PD, progressive disease; PO, by mouth; PR, partial response; R-HCVAD, rituximab + hyper-fractionated cyclophosphamide + vincristine + doxorubicin + dexamethasone; SD, stable disease.
*Data from Wang, et al.2
†Once every 12 h for six doses.
‡24-hour infusion.
§200 mg/m² IV for 2 h, then 800 mg/m² IV for 22 h.
¶Four doses.
#12 h post-methotrexate.
**24–36 h post-cytarabine
Results
• A total of 131 patients were enrolled in the WINDOW-1 study (Figure 4, Table 3).
• The median number of treatment cycles received by patients in part A and part B were seven and four, respectively, with 11 patients not continuing to part B.
Figure 4. WINDOW-1 study profile*
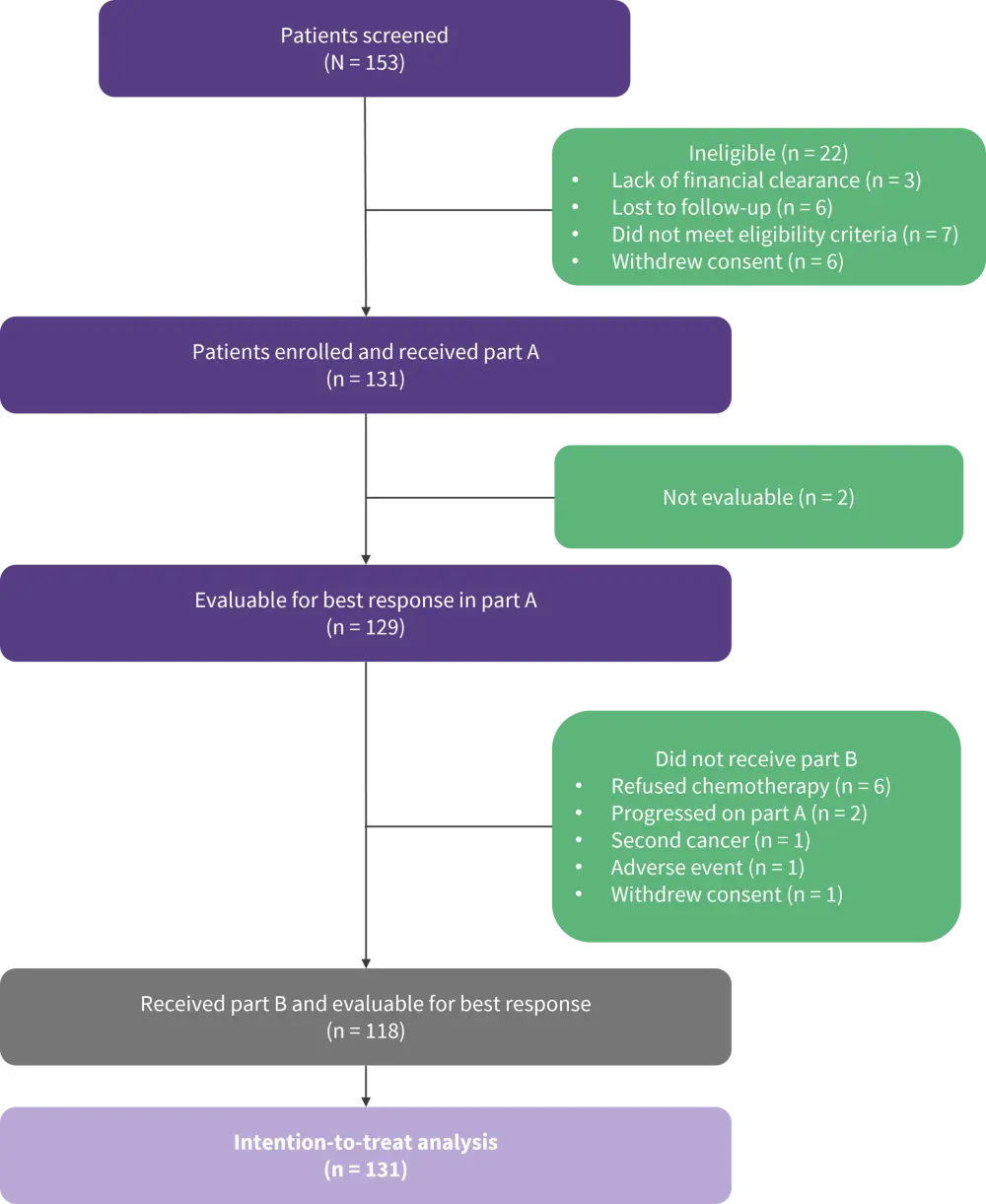
*Adapted from Wang, et al.2
Table 3. Baseline characteristics of patients enrolled in the WINDOW-1 study*
|
BM, bone marrow; ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; MIPI, Mantle Cell Lymphoma International Prognostic Index. |
|
|
Characteristic (% unless stated otherwise) |
Patients (N = 131) |
|---|---|
|
Age, years (range) |
56 (49–60) |
|
Sex |
|
|
Male |
79 |
|
Female |
21 |
|
ECOG |
|
|
0–1 |
99 |
|
2 |
<1 |
|
Bulky disease |
9 |
|
BM involvement |
88 |
|
GI involvement |
86 |
|
Simplified MIPI |
|
|
Low risk |
80 |
|
Intermediate risk |
12 |
|
High risk |
8 |
|
Biological MIPI |
|
|
Low risk |
31 |
|
Intermediate risk |
33 |
|
High risk |
36 |
|
Cytomorphology |
|
|
Classic |
88 |
|
Blastoid/pleomorphic |
12 |
|
SOX-11 |
|
|
Positive |
89 |
|
Negative |
11 |
|
Ki-67 percentage |
|
|
<30% |
45 |
|
≥30% |
44 |
|
Unavailable |
11 |
|
TP53 |
|
|
Positive |
32 |
|
Negative |
68 |
Efficacy
- Patient responses across part A and part B of the WINDOW-1 study are shown in Figure 5.
- ORR was not influenced by Ki-67 percentage, disease risk, or MCL cytomorphology. Despite comparable OS rates between patients with/without TP53 aberrations, the presence of TP53 abnormalities significantly reduced CR rates.
- At the 42-month follow-up
- 24 patients progressed and six patients died due to disease progression (n = 5) and an unknown reason (n = 1);
- median PFS and OS were not reached; and
- 3-year PFS and OS were 79% (95% CI 70–85) and 95% (89–98), respectively.
- Patients with high-risk MCL, including Ki-67 ≥50%, blastoid or pleomorphic MCL, complex karyotype, and high-risk MIPI demonstrate a significantly increased risk of disease progression. OS rates were comparable between patients with high- and low-risk disease, with the exception of patients with complex karyotypes, who exhibited inferior OS.
- BM MRD negativity by flow cytometry was observed in 65% of patients.
Figure 5. Patient outcomes following treatment in part A and part B of the WINDOW-1 study*
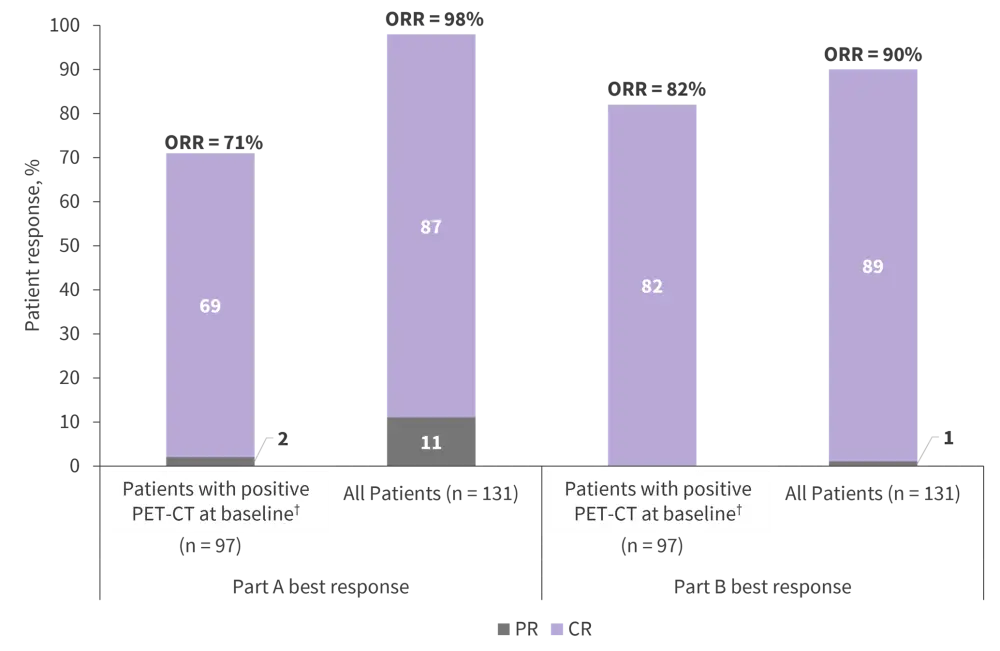
MRD, measurable residual disease; PET-CT, positron emission tomography-computed tomography.
*Data from Wang, et al.2
†All patients had baseline imaging; 30 had CT scans, and 101 had PET–CT scans.
Safety
- The most commonly observed Grade 3–4 AEs are shown in Table 4.
- In part A, dose reductions occurred in 33% of patients, but no patients discontinued treatment as a result of AEs.
- In part B, Grade 3–4 AEs resulted in chemotherapy dose reductions in 22% of patients, and four patients discontinued treatment due to serious AEs.
- There was one death during study, which was not attributed to the study drugs.
Table 4. Common Grade 3–4 AEs observed in the WINDOW-1 study*
|
AE, adverse event. |
||||
|
AE, % |
Part A (n = 131) |
Part B (n = 131†) |
||
|---|---|---|---|---|
|
Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
|
|
Hematologic |
||||
|
Anemia |
4 |
— |
17 |
— |
|
Lymphocytopenia |
9 |
5 |
23 |
50 |
|
Leukocytopenia |
2 |
— |
10 |
22 |
|
Thrombocytopenia |
9 |
— |
6 |
24 |
|
Neutropenia |
4 |
2 |
5 |
14 |
|
Nonhematologic |
||||
|
Skin rash |
12 |
— |
3 |
— |
|
Infection |
8 |
— |
4 |
— |
|
Fatigue |
8 |
— |
19 |
— |
|
Elevated liver enzymes |
4 |
— |
9 |
2 |
|
Myalgia |
6 |
— |
9 |
— |
WINDOW-1 conclusions
In the WINDOW-1 study, ibrutinib–rituximab induction with subsequent alternating R-HCVAD and methotrexate–cytarabine consolidation demonstrated impressive ORR and CR rates in young patients with treatment-naïve MCL. Induction alone resulted in an ORR of 98%, and outcomes did not differ significantly between patients who received at least four cycles of chemotherapy and those not completing part B. Median OS and PFS were not reached at the 42-month follow-up.
Data from the study demonstrate the feasibility, efficacy, and favorable toxicity profile of chemotherapy-free induction followed by reduced-exposure consolidation in the frontline MCL setting; however, the authors note that the transition from chemotherapy to chemotherapy-free regimens should be approached with caution.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

