All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Ibrutinib with bendamustine and rituximab in R/R CLL/SLL: Final 5-year results from the HELIOS trial
Visual abstract
Final 5-year results from the Phase 3 HELIOS trial
If you would like to download this visual abstract, click below.
Introduction
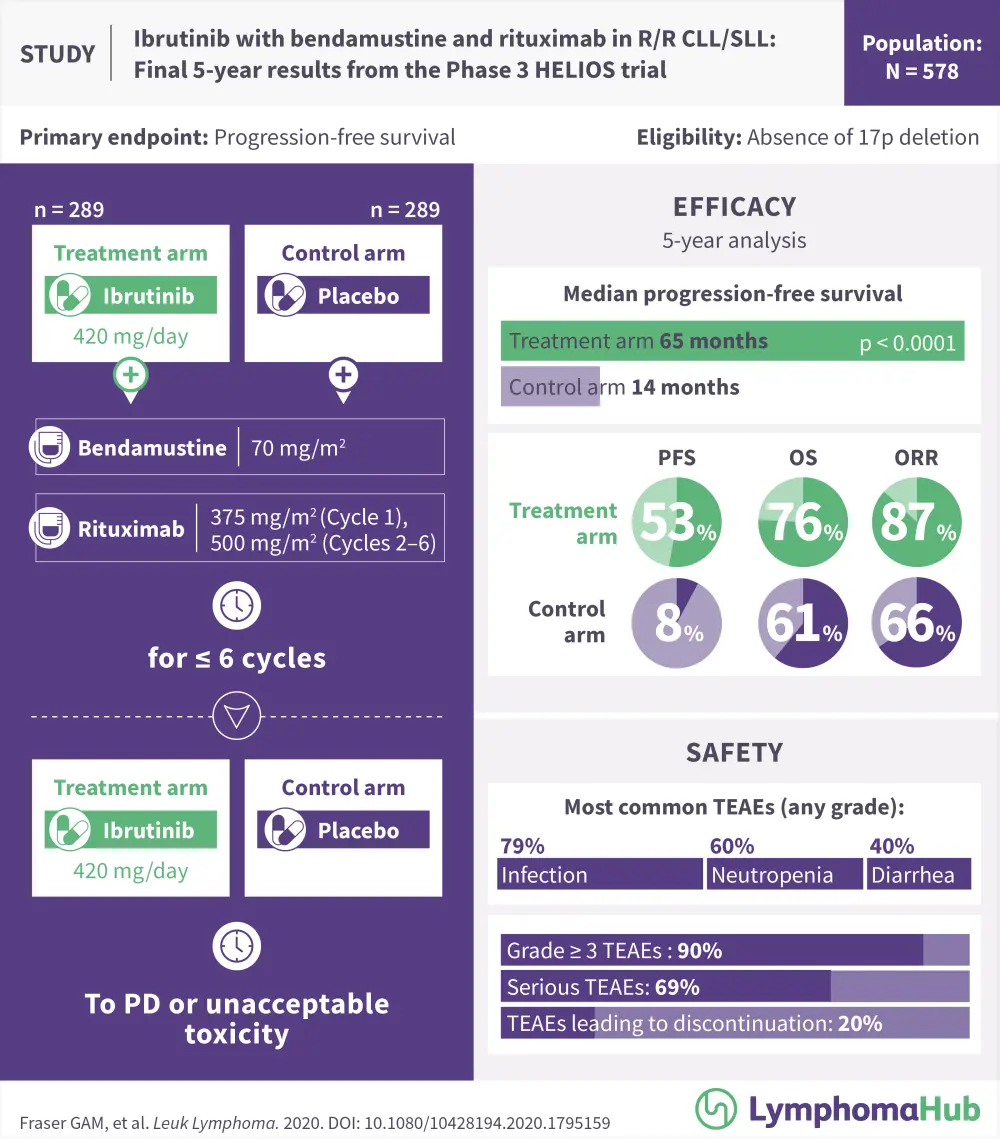
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor that has been approved to treat various B-cell malignancies in adults. It is now one of the preferred treatments for patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).1,2 The phase III HELIOS trial (NCT01611090) was set up to investigate the efficacy of ibrutinib with bendamustine and rituximab (BR) compared with placebo and BR. The 3-year results were covered by the Lymphoma Hub in 2018 and can be found here. The final 5-year results of the HELIOS trial were reported by Graeme Fraser and colleagues in Leukemia and Lymphoma1 and are summarized below.
Patients and methods
HELIOS was a randomized, double-blind, placebo-controlled phase III trial of 578 patients with relapsed/refractory (R/R) CLL/SLL.
Eligible patients were ≥ 18 years with active CLL/SLL as defined by the International Workshop on Chronic Lymphocytic Leukemia 2008 criteria.2
Further inclusion criteria:
- R/R disease following ≥ 1 line of previous therapy
- Eastern Cooperative Oncology Group performance status 0−1
- Measurable lymph node disease (> 1.5 cm) by computed tomography
Patients with chromosome 17p deletion were excluded from this trial, as they are known to experience poor responses when treated with BR.
Study design
Patients were randomly assigned (1:1) to the ibrutinib + BR group or the placebo + BR group.
Patients were treated with:
- Ibrutinib: 420 mg daily, plus
- Bendamustine: 70 mg/m2 intravenously on Days 2–3 in Cycle 1 and Days 1–2 in Cycles 2–6, and
- Rituximab: 375 mg/m2 on Day 1 of Cycle 1 and 500 mg/m2 on Day 1 of Cycles 2–6; or
- BR with placebo
Treatment was continued until disease progression or unacceptable toxicity.
In March 2015, following the advice of the Data Safety Monitoring Board, the study was unblinded and placebo treatment was ended. The recording of treatment-emergent adverse events (TEAEs) ceased at this time too. A total 183 of 298 patients (63.3%) from the placebo arm were allowed to crossover to the ibrutinib treatment arm after confirmed disease progression and follow-up data were recorded.
The endpoints investigated were investigator-assessed progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and safety. PFS2 (time from randomization to disease progression/death/start of subsequent antineoplastic therapy if progression not recorded) was also measured.
Key results
In the final analysis, the overall median follow-up was 63.7 months (95% CI, 62.8−64.3; range, 0.1−74.5) and was similar between arms.
The median time on treatment for the ibrutinib + BR group (n = 287) was 55.7 months (range, 0.2−72.9), compared to 14.3 months (range, 0.2−30.6) for the placebo + BR arm (n = 287; placebo arm was discontinued at interim analysis).
The key outcomes are shown below in Table 1. In brief, at 60 months, PFS for the ibrutinib + BR group was 52.7% compared with 8.2% for the placebo group. Despite the crossover (63.3% of patients), the 5-year OS advantage of ibrutinib was maintained (HR, 0.611; 95% CI, 0.455−0.822; p = 0.001). Investigator-assessed ORR was 87.2% for ibrutinib + BR and 66.1% for placebo + BR (p < 0.0001), and responses were found to increase over time. Complete response/complete response with incomplete bone marrow recovery increased from 21.5% in the interim 3-year analysis to 40.8% at 5 years.
In the ibrutinib + BR arm, 28.7% of patients were measurable residual disease (MRD) negative in peripheral blood or bone marrow, which was similar to the 3-year interim results (26.3%).
Table 1. Key outcomes for the ibrutinib + BR group compared with the placebo + BR group1
|
BR, bendamustine and rituximab; CI, confidence interval; HR, hazard ratio; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PFS2, time from randomization to disease progression/death or start of new therapy if progression not recorded |
|||
|
Key outcomes |
Ibrutinib + BR (n = 289) |
Placebo + BR (n = 289) |
HR (95% CI); p value |
|---|---|---|---|
|
Median PFS, months |
65.1 |
14.4 |
0.229 (0.183–0.286); p < 0.0001 |
|
60-month PFS, % |
52.7 |
8.2 |
— |
|
Median OS, months |
NR |
NR |
— |
|
60-month OS, % |
75.5 |
61.2 |
— |
|
Median PFS2, months |
NR |
63.0 |
0.594 (0.453-0.779; p = 0.0001 |
|
ORR, % |
87.2 |
66.1 |
p < 0.0001 |
Safety
Safety data were reported only from the ibrutinib + BR arm, so no comparisons with the placebo group were possible. At 5 years, a total of 90.2% of patients had at least one Grade ≥ 3 TEAE and 20.2% had TEAEs sufficient to lead to ibrutinib discontinuation.
Infection was the most common TEAE (any grade), occurring in 78.7% patients, followed by neutropenia (59.9%), diarrhea (40.4%), anemia (25.8%), hypertension (16.7%), and atrial fibrillation (11.8%). Major hemorrhage of any grade occurred in 5.6% of patients, including three patients (1.0%) with Grade 5 hemorrhagic events. The occurrence of TEAEs generally decreased over time.
Overall, in the ibrutinib + BR arm, 25.8% of patients died. Of those, 35 deaths were due to adverse events (11 of which were treatment related), 17 were due to disease progression, and 22 were due to other reasons.
Conclusion
The final 5-year results of the HELIOS trial confirm the benefit of ibrutinib in combination with BR in R/R patients with CLL/SLL and suggest a prolonged survival benefit over the placebo + BR group. The safety and toxicity findings were consistent with previous data and support the continued use of ibrutinib in this group of patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

