All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ICML 2017 | Interim results of the phase II JULIET and primary results of the pivotal ZUMA-1 trials: CAR-T cell therapy for R/R DLBCL – Joint Session
On the first day of the 14th International Conference on Malignant Lymphoma (ICML) taking place in Lugano, Switzerland, the LH attended an AACR-ICML Joint Session on Cancer Immunotherapy.
The session was co-chaired by Franco Cavalli (Ente Ospedaliero Cantonale, Bellinzona, Switzerland) and Margaret Foti (Chief Executive Officer, American Association for Cancer Research).
- Both JULIET and ZUMA-1 met their primary endpoints
- Target dose: for JULIET was 1–5 x 108 versus 2 x 106 in ZUMA-1
- Median OS was not reached in both studies
- Best ORR was higher in ZUMA-1 (82%; CR = 49%) compared to JULIET (59%; CR = 43%)
- Grade ≥3 CRS and NE was higher in ZUMA-1 (34% and 28%) versus JULIET (26% and 13%)
- Thus, higher proportions of patients received tocilizumab or steroids in ZUMA-1 (43% and 27%) compared to JULIET (16% and 11%)
Abstract 007
The first talk in this session was given by Stephen J. Schuster, MD, from the Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA, and was focused on interim analysis of the JULIET study (NCT02445248).
Schuster began by emphasizing that patients with R/R DLBCL have a poor outcome. Patients refractory to chemotherapy or who experience relapse within one year of ASCT are unlikely to respond to subsequent therapy (CR = 8%; PR = 18%; Crump et al. ASCO. 2016. Abstract #7516). Furthermore, patients who fail second-line salvage therapy have particularly poor prognosis (median OS = 4.4 months; 1-year OS = 23%; 2-year OS = 15.7%; Van Den Neste et al. BMT. 2016).
CTL019 is a Chimeric Antigen Receptor (CAR) T-cell therapy, which has been shown to achieve a high rate of durable CRs and manageable toxicity in a single-center, phase II trial at the University of Pennsylvania in adult patients with R/R DLBCL (Schuster et al. ASH. 2015. Abstract #183; Schuster et al. ASH. 2016. Abstract #3026). None of the patients who achieved a CR at 6 months has relapsed. Duration of response after a median follow-up of 23.3 months was 85.7% (95% CI, 33.7–97.9%).
|
|
Month 3 |
Month 6 |
|---|---|---|
|
ORR |
47% |
47% |
|
CR |
20% |
40% |
|
PR |
27% |
7% |
JULIET is a phase II, single-arm, multicenter trial aiming to evaluate the efficacy and safety of CTL019 in adult patients with R/R DLBCL.
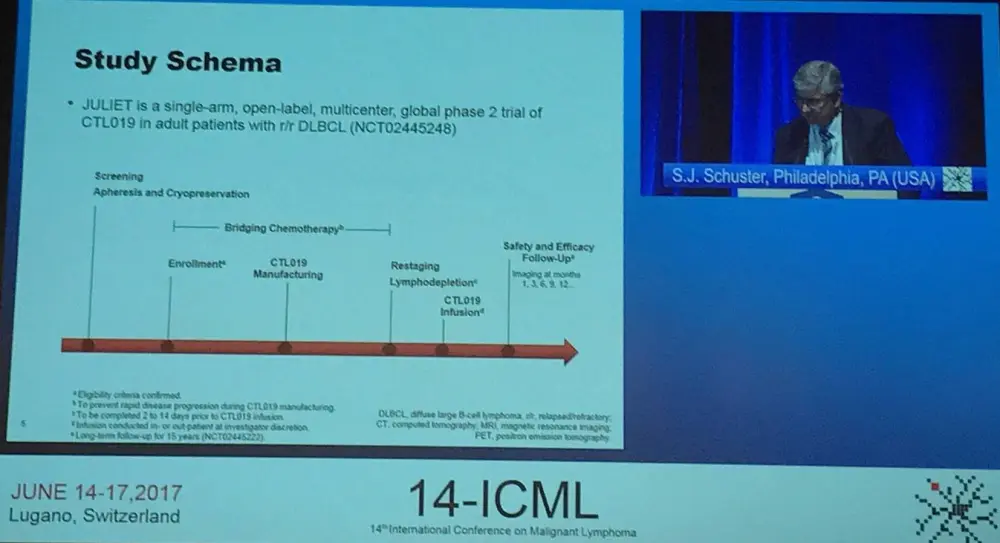
Industry-manufactured CAR-T cells were administered patients located at 27 different centers across 10 countries in North America, Europe, Australia, and Asia using a global supply chain. Autologous T-cells were transduced with a lentiviral vector encoding an anti-CD19 CAR, expanded, cryopreserved, shipped, and infused at study sites. Best ORR (CR + PR) was the primary endpoint. Secondary outcome measures are DoR, OS, and safety. Target dose was (range) 1–5 x 108 CTL019 transduced cells.
Key eligibility criteria was listed by Schuster as:
- Aged 18 years or older
- Central confirmation of DLBCL by histology
- R/R DLBCL measurable disease at enrollment
- Two or more prior lines of therapy for DLBCL
- Failed or ineligible for ASCT and no prior allo-SCT
- ECOG PS of 0–1 at screening
- Adequate organ function
- No prior anti-CD19 therapy
- No active CNS involvement
Enrollment began in July 2015 and recruited 141 patients; the data cut-off was December 2016. Overall, 43 patients discontinued before infusion (9 due to inability to manufacture and 34 related to patient status). Of the remaining patients and following restaging, bridging therapy, and lymphodepleting chemotherapy (fludarabine 25mg/m2 / cyclophosphamide 250mg/m2/day × 3 days or bendamustine 90mg/m2/day × 2 days), 85 patients were infused with a single dose of CTL019 transduced cells (median, 3.1 × 108; range, 0.1–6.0 × 108 cells). These 85 patients were evaluated for safety and 51 were evaluated for response (completed 3 or more months of follow-up or discontinued earlier). Median time from infusion to data cutoff was 3.7 months.
Median age was 56 years (range, 24–75) and median number of previous lines of anti-neoplastic therapy was 3 (range, 2–7). Over half (51%) of patients had prior ASCT. Out of all 85 patients, 76 received bridging chemotherapy.
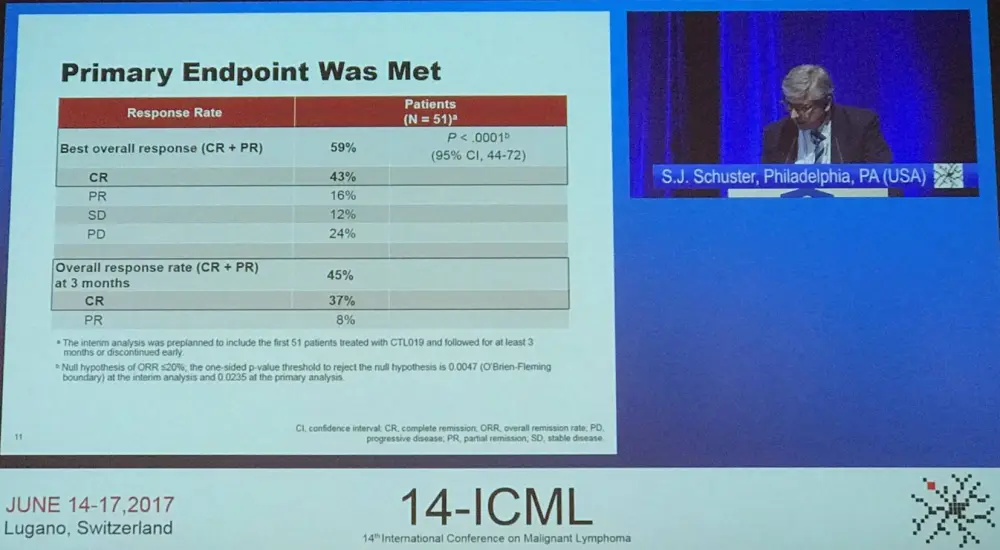
All patients who had achieved CR at 3 months remained in CR at data cut-off. Efficacy was observed across prognostic subgroups. Median DoR and OS were not reached, and 79% of patients were relapse free at 6 months. Quantitative PCR detected CTL019 in the peripheral blood of responders for up to 355 days. No cases of cerebral edema or deaths due to CTL019 or Cytokine Release Syndrome (CRS) were reported.
|
AE of special interest |
All grade (%) |
Grade 3 (%) |
Grade 4 (%) |
|---|---|---|---|
|
Cytokine Release Syndrome |
57 |
17 |
9 |
|
Infections |
27 |
12 |
1 |
|
Cytopenias not resolved by day 28 |
26 |
13 |
8 |
|
Neurologic events |
21 |
9 |
4 |
|
Febrile neutropenia |
14 |
13 |
1 |
|
Tumor Lysis Syndrome |
1 |
1 |
0 |
The median time to onset of CRS was 3.0 days (range, 1–8) and median duration of CRS was 7.0 days (range, 3–34). Nearly one-quarter (24%) of patients who experienced CRS were admitted to intensive care, and 29% of patients experienced hypotension that required intervention. 16% of patients received tocilizumab and 11% received corticosteroids for CRS management. 3 patients died from disease progression within 30 days of infusion.
Stephen J. Schuster concluded his part of the session with a succinct summary slide:

This planned interim analysis of the phase II JULIET trial of CTL019 CAR-T therapy in adults with R/R DLBCL indicates that this therapy achieves high response rates and durable CRs as observed in the previous single-center experience. The feasibility of centralized manufacturing was also demonstrated. AEs could also be effectively and reproducibly managed.
Abstract 008
The second talk during this session discussed primary results from the pivotal phase I/II ZUMA-1 trial (NCT02348216) of KTE-C19 in patients with refractory DLBCL and was presented by Sattva S. Neelapu from The University of Texas MD Anderson Cancer Center, Houston, USA.
This talk was originally given by Frederick L. Locke, MD, from the H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, at AACR 2017; which the LH reported on.
Once again, the significant unmet need in refractory aggressive NHLs was emphasized. NHL is the most common hematologic malignancy in the US and outcomes in patients with refractory aggressive disease are poor: Neelapu also referred to the SCHOLAR-1 meta-analysis, which found an ORR of just 26% and a median OS of 6.6 months.
KTE-C19 is also an autologous CAR-T therapy which utilizes CDζ/CD28-based signaling that identifies and eliminates CD19-expressing cells. ZUMA-1 was a multicenter study which aimed to assess the safety and efficacy of KTE-C19 in patients with refractory aggressive NHLs.
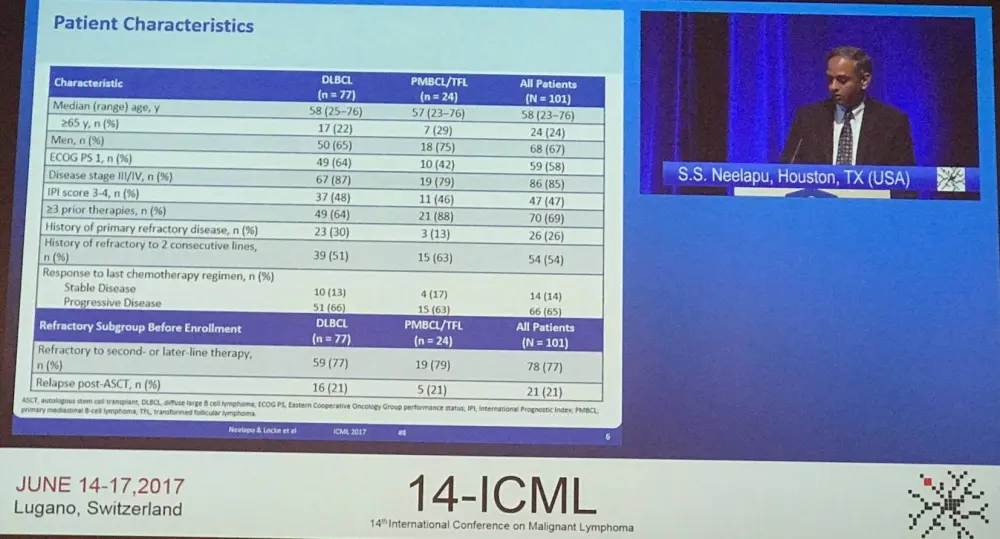
Overall, 111 patients were enrolled from 22 sites, but 10 patients were not treated due to SAE (5 before conditioning, 2 after conditioning), no measurable disease (2 before conditioning), and product unavailable (1 before conditioning). No bridging therapy was allowed and conditioning consisted of cytarabine 500mg/m2 and fludarabine 30mg/m2 x 3 days. The target dose of KTE-C19 was 2 x 106 cells/kg (n=101). Ninety-two patients were included in the primary analysis, this increased to 101 for the Modified Intent to Treat (mITT) analysis. Data cut-off was January 2017, and median follow-up was 8.7 months. The manufacturing success rate was 99% (110/111 patients) and a 17-day average turnaround time from apheresis to delivery at clinical site was reported. The majority (91%) of patients received KTe-C19. Median age was 58 years (range, 23–76), 67% of patients were male, 85% had stage III–IV disease, 47% were IPI 3–4, 77% were refractory to second-line or greater therapy, and 21% relapsed within one year of last therapy.
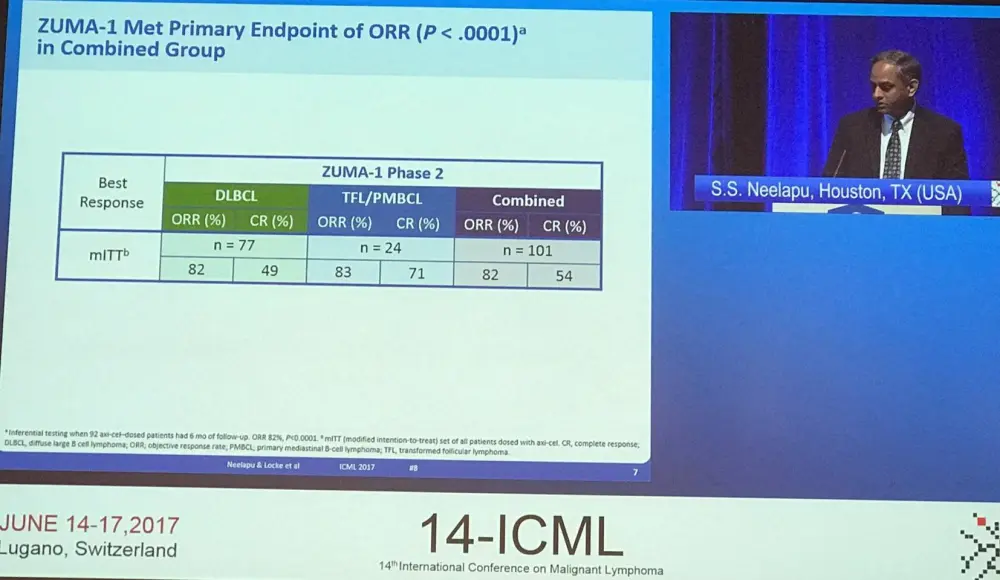
Responses were consistent across key covariates, including between ABC (76% ORR, 59% CR) and GCB (88% ORR, 57% CR) subtypes. Responses have been found to be durable, with 44% ongoing at 8.7 months of follow-up. The median DOR was 8.2 months overall and not reached for patients who achieved a CR. PFS rates at 6 months were consistent across key covariates also; median PFS was 5.9 months (95% CI, 3.4–9.8). Median OS was not reached; 80% of patients remained alive at 6 months compared to 55% of patients in the SCHOLAR-1 analysis.
Following this, Neelapu listed the most frequent grade 3 or higher treatment-emergent AEs, then gave a summary of all AEs:
|
Grade ≥3 AE, n (%) |
N=101 |
|---|---|
|
Anemia |
43 (43) |
|
Neutropenia |
39 (39) |
|
Neutrophil count decreased |
32 (32) |
|
Febrile neutropenia |
31 (31) |
|
White blood cell count decreased |
29 (29) |
|
Thrombocytopenia |
24 (24) |
|
Encephalopathy |
21 (21) |
|
Lymphocyte count decreased |
20 (20) |
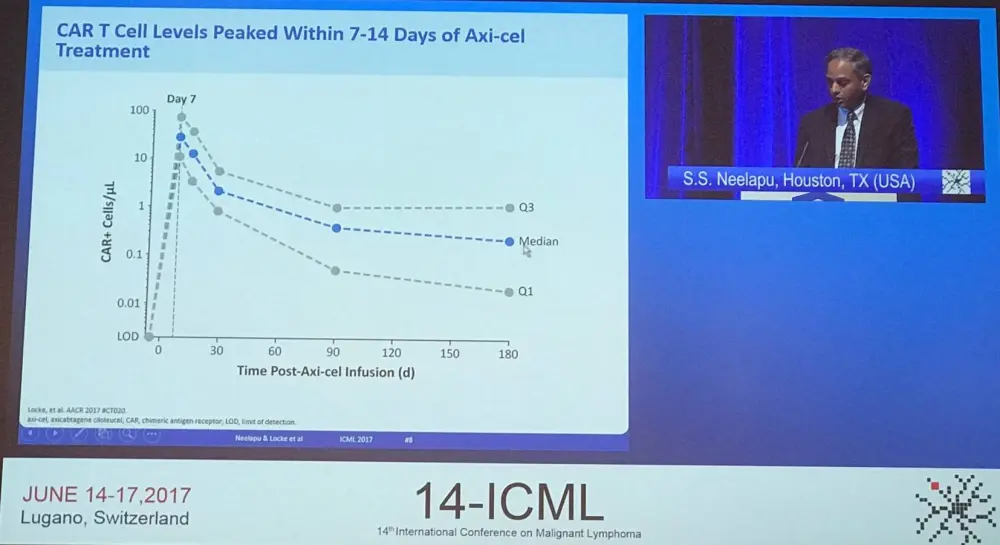
Grade ≥3 AEs in the interim analysis (n=62) had an incidence of 95% (59/62), the same as that observed in the primary analysis (95/101). The incidence of grade ≥3 CRS (18% and 34%) and Neurologic Events (NE; 13% and 28%) decreased from the interim analysis to the primary analysis. CRS and NE were in most cases reversible, all CRS events have resolved except one case of HLH and one case of cardiac arrest. All NEs have resolved except one grade 1 memory impairment. In terms of management of CRS, 43% of patients received tocilizumab and 27% received steroids. The administration of tocilizumab/steroids did not impact on responses but was associated with higher median peak CAR-T cell levels (P = 0.0011; P = 0.0618). Compared to the interim analysis, no new KTE-C19-related grade 5 AEs have been observed.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

