All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
iwCLL 2017 | Life after ibrutinib: pre-clinical and clinical studies
The sixth session at this year’s iwCLL was titled “Genetic Changes Involved in the Progression and Evolution of CLL”, and was jointly chaired by Christopher Vakoc (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, New York) and Nicole Lamanna (New York Presbyterian and Columbia University Medical Center, New York, New York).
The presentation titled “Life After Ibrutinib: Pre-clinical and clinical studies” was given during this session by Professor John C. Byrd from The Ohio State University, Columbus, Ohio.
The presentation began with John Byrd describing three main types of CLL that he would then go one to describe as the good (ibrutinib-sensitive CLL), the bad (ibrutinib-resistant CLL), and the ugly (Richter’s transformation).
- Survival outcomes for R/R CLL after ibrutinib treatment is problematic
- After ibrutinib initiation:
- de novo CLL 5-year PFS = 92% vs. 43% in R/R CLL pts (Susan O’Brien, ASH 2016)
- 5-year OS in de novo CLL = 92% vs. 57% in R/R CLL pts (Susan O’Brien, ASH 2016)
- ibrutinib is highly-effective in first-line treatment
- long-term side effects of ibrutinib treatment were ‘mild to modest’, allowing most patients to remain on treatment
- Ibrutinib resistance can occur via:
- Mutations in BTK at C481 resulting in impaired ibrutinib binding efficiency
- Gain of function BCR signaling mutations in PLCG2 at R665, L845, or S707 (unpublished)
- Early resistance to ibrutinib is rare, more frequently presents as Richter’s transformation
- Richter’s transformation occurs earlier in CLL progression, not later:
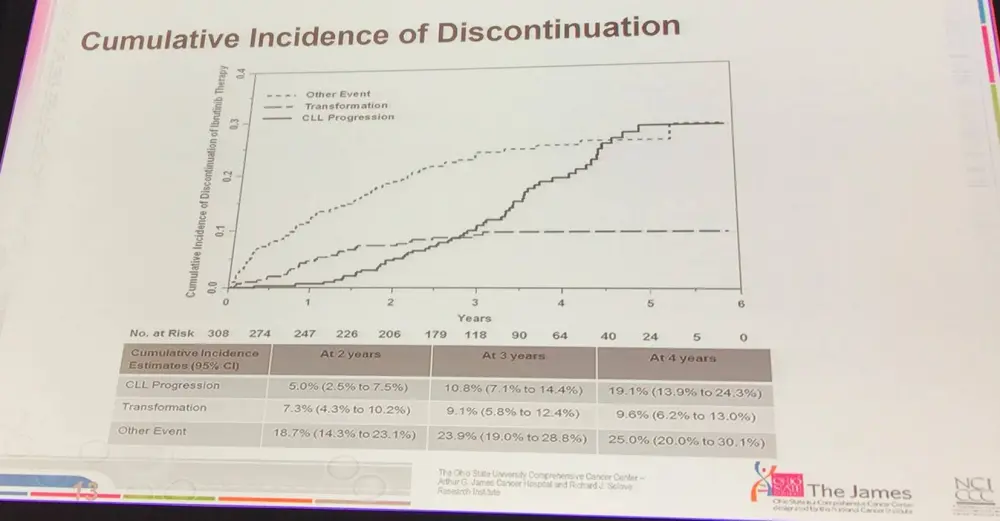
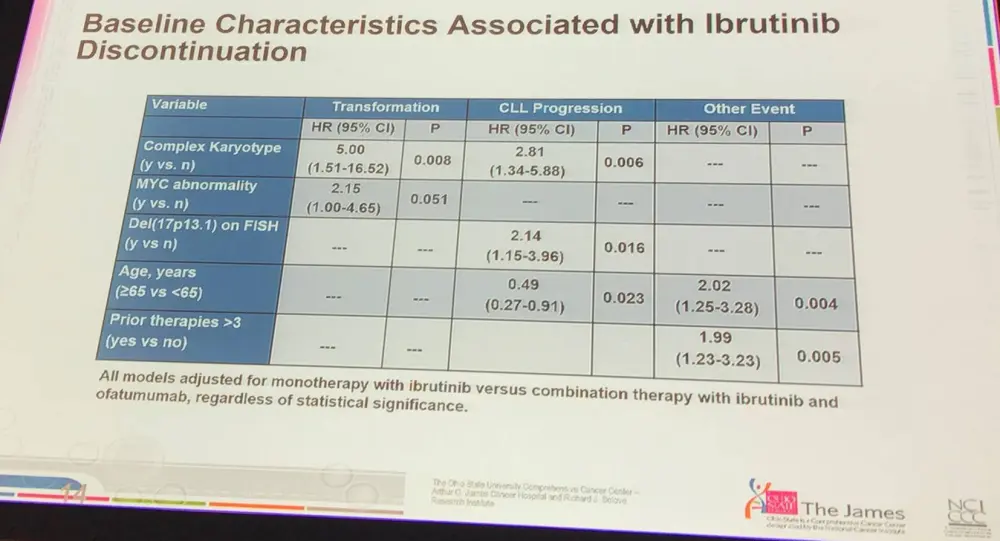
- Complex karyotype at baseline was significantly associated with transformation; myc abnormalities almost were significant
- Age was a significant factor for discontinuing treatment suggesting that new treatments are needed for older pts (≥65)
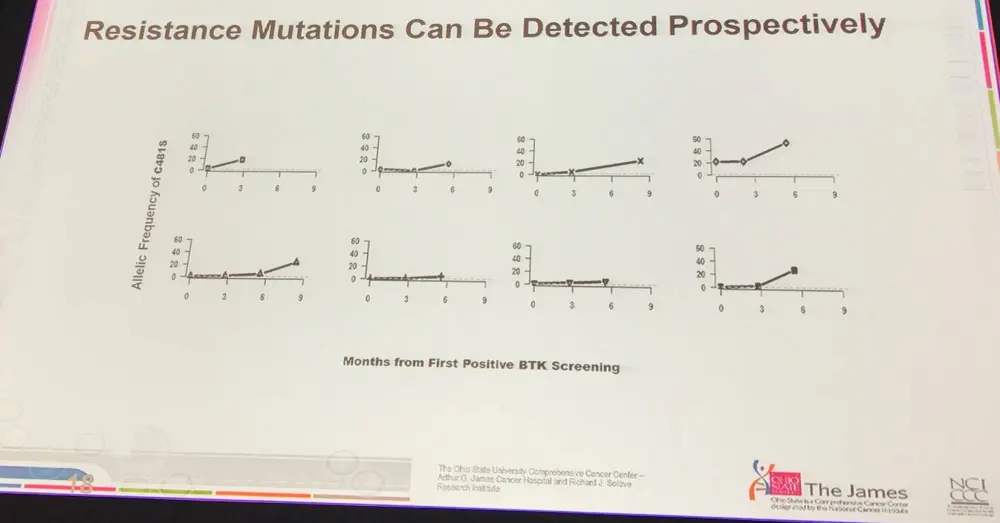
- BTK and PLCG2 mutations occurred ~9 months before relapse with sensitive assays, such as Ion Torrent
- Pts which relapse after ibrutinib present with resistant disease
- Possible to detect resistant mutations in BTK prospectively
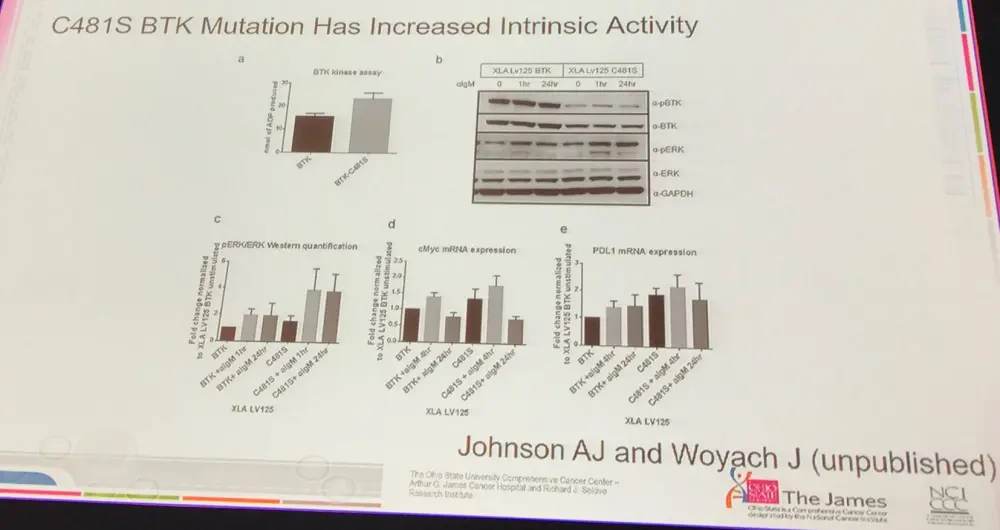
- Resistance mutations are still there after salvage therapy, and upon therapy cessation the clones are still present indicating that they are genuine gain of function mutations (BTK C481S)
- Strategies for targeting ibrutinib resistance include:
- Reversible BTK inhibitors which don’t use the C481 binding site
- CAR-T cells
- Venetoclax
- Other antibody or immunotherapies for CLL
- HSP-90 inhibitor to reduce BTK levels
- May be possible to treat PLCG2 mutated CLL with entospletinib
- The advice generally is to enroll these high-risk patients onto clinical trials for more effective therapies
- New therapies in early clinical trials include:
- SNS-062, a selective BTK inhibitor in phase I trials (Fabian, C. and Johnson, A.J. AACR 2017)
- GDC0853, a selective BTK inhibitor whose phase I trial was discontinued for company priority reasons (Reif, S. and Woyach, J. ASCO 2016)
- ARQ531, a less selective BTK inhibitor, initiating phase I trials now to be reported at ASH 2017
- ARQ531
- Broad inhibitor of TEC and SRC family kinases, with a long half-life in vivo and favorable in vivo toxicity
- Early data showed superior in vivo activity compared with ibrutinib in TCL1 model, and in Richter’s transformation model
- Antagonizes cellular migration in primates without high toxicity
- ARQ531 was shown to inhibit BTK-C481S similarly to wild-type BTK
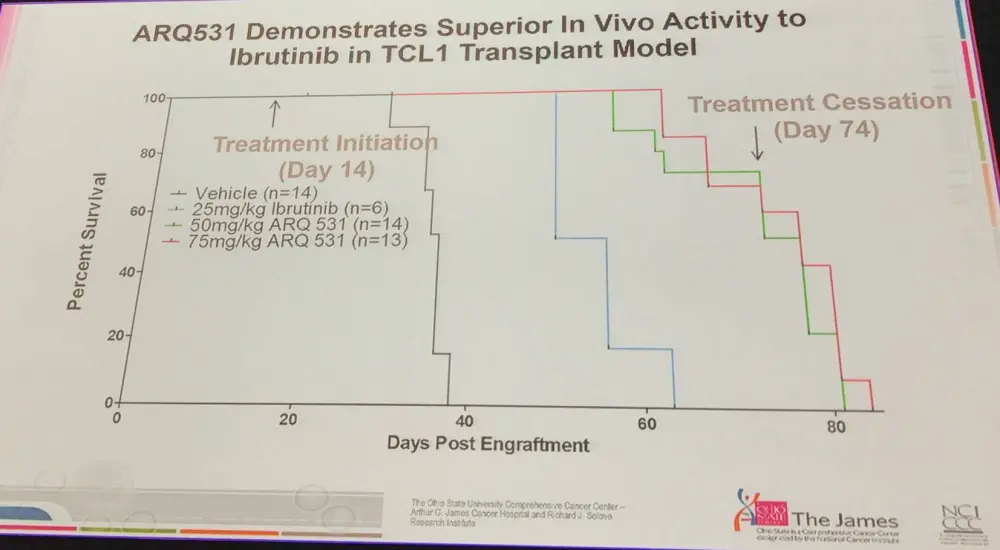
- Strategies for secondary resistance include detecting treatment resistant clones earlier, and adding in a second agent alongside ibrutinib
Richter’s syndrome:
- CLL to Richter’s transformation biology is poorly understood
- Chemotherapy to treat Richter’s syndrome is generally not effective, or only provides a short response
- PD1 blockade may work against Richter’s syndrome but not CLL, but may be useful
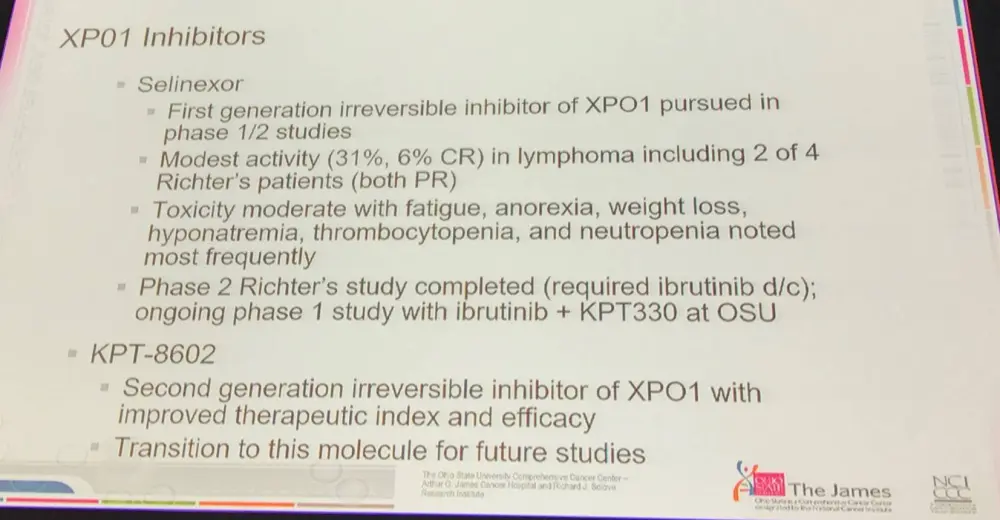
- XPO1 (Exportin1) expression correlated with poor prognosis in cancer, and is overexpressed in CLL
- XPO1 is implicated in multiple cell death pathways, and is therefore an attractive target
- Selinexor and KPT-8602 are irreversible first and second generation XPO1 inhibitors respectively and are detailed below:
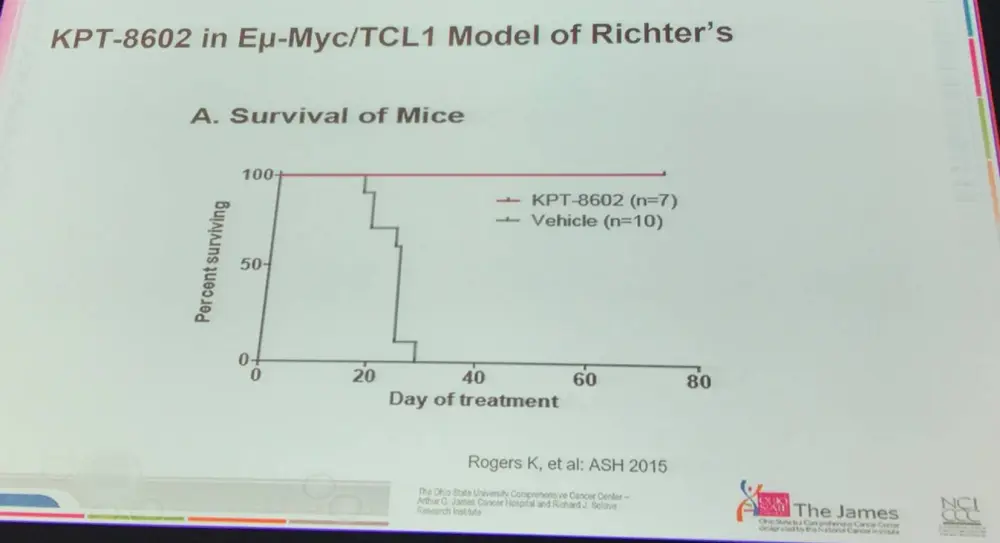
- In vivo data of KPT-8602 in Eu-Myc/TCL1 mouse model of Richter’s syndrome showed curative potential of this approach
- PLX51107A Bromodomain Extra-Terminal protein family (BET) inhibitor, targeting BRD4 which is expressed in CLL, is about to enter trials in CLL and Richter’s transformation
John Byrd concluded his presentation that while treatment strategies have improved in first-line CLL (the Good), the picture is less positive for ibrutinib-resistant patients (the Bad), and Richter’s transformation remains ‘Ugly’, but there is progress being made on the treatment options for these patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

