All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Lisocabtagene maraleucel as second-line therapy for adult patients with LBCL: Primary analysis of the phase III TRANSFORM trial
For patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL), platinum-based immunochemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT) has been the standard of care (SOC) second-line treatment protocol for many years. However, outcomes for these patients have historically been poor, with less than 30% of patients being cured. Recent data have indicated that these patients may benefit from chimeric antigen receptor (CAR) T-cell therapies as second-line treatment.
Lisocabtagene maraleucel (liso-cel) is an CD19-directed, autologous CAR T-cell product administered as equal doses of CD8+ and CD4+ CAR+ T-cells. Recent research has examined the role liso-cel may play in treating patients with R/R DLBCL as part of the TRANSFORM trial (NCT03575351).
Here, we summarize results from a primary analysis of the ongoing phase III TRANSFORM trial comparing the efficacy and safety of liso-cel to SOC in adult patients with high-risk, R/R, aggressive LBCL, presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition. These results were also published in Blood in December 2022.
Study design1
TRANSFORM is an ongoing, randomized, open label phase III trial of liso-cel vs SOC second-line therapy in adult patients with R/R LBCL. A total of 184 patients aged 18–75 years were selected for trial enrollment. Key enrollment criteria included positron emission tomography positive LBCL per Lugano 2014 criteria, R/R disease after CD20 antibody and anthracycline-containing first-line therapy, Eastern Cooperative Oncology Group Performance Status ≤1, adequate organ function, and eligibility for high-dose chemotherapy and ASCT.
All patients underwent leukapheresis before 1:1 randomization to liso-cel or SOC treatment arms. Patients randomized to liso-cel received lymphodepleting chemotherapy (fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 daily for 3 days) followed by liso-cel infusion at a dose of 100 × 106 CAR T-cells. Patients randomized to SOC received three cycles of immunochemotherapy with response evaluated by positron emission/computed tomography scan after three cycles. Patients achieving a complete or partial response (CR or PR) moved on to high-dose chemotherapy and ASCT.
The primary efficacy endpoint was event-free survival (EFS). Key secondary efficacy endpoints included CR rate, progression-free survival (PFS), and overall survival (OS). Exploratory efficacy endpoints included EFS, CR rate, PFS, OS, overall response rate, and duration of response for crossover patients.
Safety endpoints included the type, frequency, and severity of adverse events (AE), serious AEs, and laboratory abnormalities.
Results
Efficacy1,2
Key efficacy outcomes are shown in Figure 1.
Figure 1. Efficacy outcomes for liso-cel versus SOC*
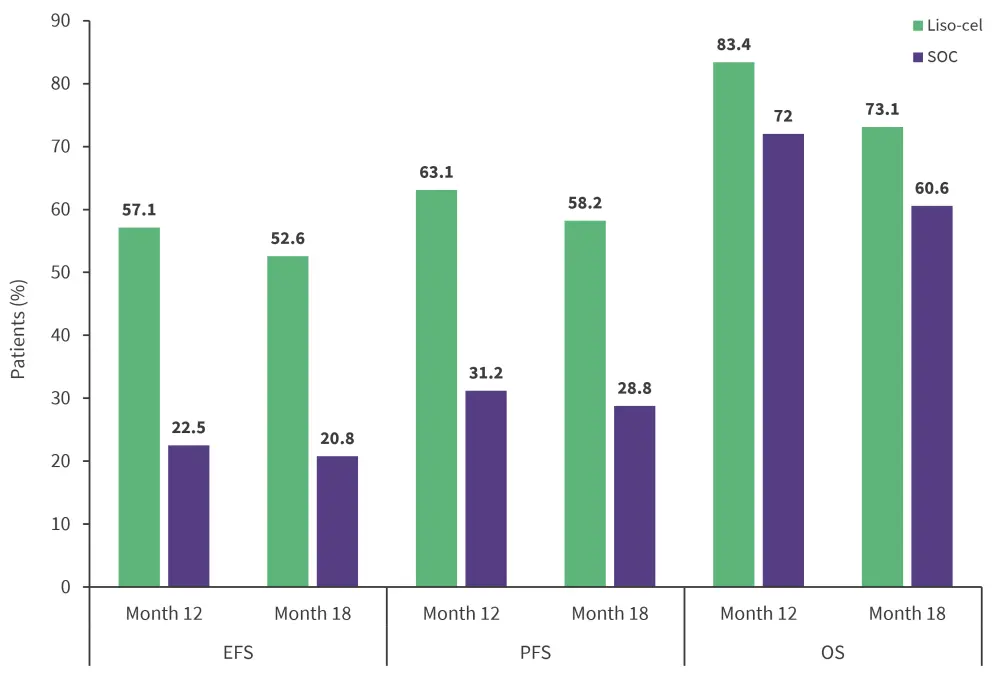
EFS, event-free survival; liso-cel, lisocabtagene maraleucel; OS, overall survival; PFS, progression-free survival; SOC, standard of care.
*Adapted from Abramson, et al.1
- Median EFS was not reached for liso-cel vs 2.4 months for SOC
- EFS was found to favor liso-cel across all subgroups (prior response status, age, sex, Eastern Cooperative Oncology Group Performance Status, lactate dehydrogenase, prior computed tomography response status, disease subtype).
- CR rate was 74% for liso-cel versus 43% for SOC
- Median PFS was not reached for liso-cel versus 6.2 months for SOC
- Median OS was not reached for liso-cel versus 29.9 months for SOC.
- The secondary efficacy endpoints overall response rate, duration of response, and duration of CR were also shown to favor liso-cel
Safety1,2
- The most common treatment emergent AEs of any grade were neutropenia, anemia, thrombocytopenia, and nausea. The most common Grade ≥3 AEs in were neutropenia, thrombocytopenia, and anemia.
- In total, 66 patients died during the trial (28 in the liso-cel arm and 38 in the SOC arm), with the most commonly reported cause of death being disease progression.
- Rates of any-grade cytokine release syndrome (CRS) and neurological events were 49% and 11%, respectively, with Grade 3 CRS occurring in 1% and NEs occurring in 4% of patients.
- Severe infections were reported in 15% of patients in the liso-cel arm and 21% in the SOC arm.
Conclusion
After a median follow-up of 17.5 months, this primary analysis of the TRANSFORM study confirms the superiority of liso-cel over SOC, with significant improvements in EFS, CR, and PFS observed in patients treated with liso-cel. The observed safety profile was comparable to previous studies, with low rates of any-grade and severe CRS and neurological events reported. These data further support the use of liso-cel as a second-line treatment for patients with primary refractory or early relapsed LBCL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


