All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
MRD-guided venetoclax plus ibrutinib for R/R CLL: results from the phase II HOVON141/VISION trial
The role of minimal residual disease (MRD)-guided treatment decisions in chronic lymphocytic leukemia (CLL) has not yet been well defined and current protocols are based on continuous or fixed-duration treatment, regardless of response. Below, we summarize the key safety and efficacy results of MRD-guided stop and start of venetoclax plus ibrutinib treatment for patients with relapsed/refractory (R/R) CLL from the phase II, open-label, randomized HOVON141/VISION trial (NCT03226301) published by Kater, et al., in Lancet Oncology in 2022.1
Study design
The HOVON141/VISION trial, previously reported on the Lymphoma Hub, was conducted in 47 European hospitals. Eligible patients were
- aged ≥18 years;
- had previously treated CLL;
- with or without TP53 aberrations;
- not previously exposed to Bruton’s tyrosine-kinase inhibitors or BCL2 inhibitors;
- had a creatinine clearance rate of ≥30 mL/min; and
- required treatment according to the International Workshop on Chronic Lymphocytic Leukemia 2018 criteria.2
Patients with undetectable MRD (uMRD) after 15 cycles of ibrutinib plus venetoclax were randomly assigned (1:2) to continue or stop ibrutinib treatment (Figure 1). The primary endpoint was 27-month progression-free survival (PFS) in the treatment cessation group.
Figure 1. study design*
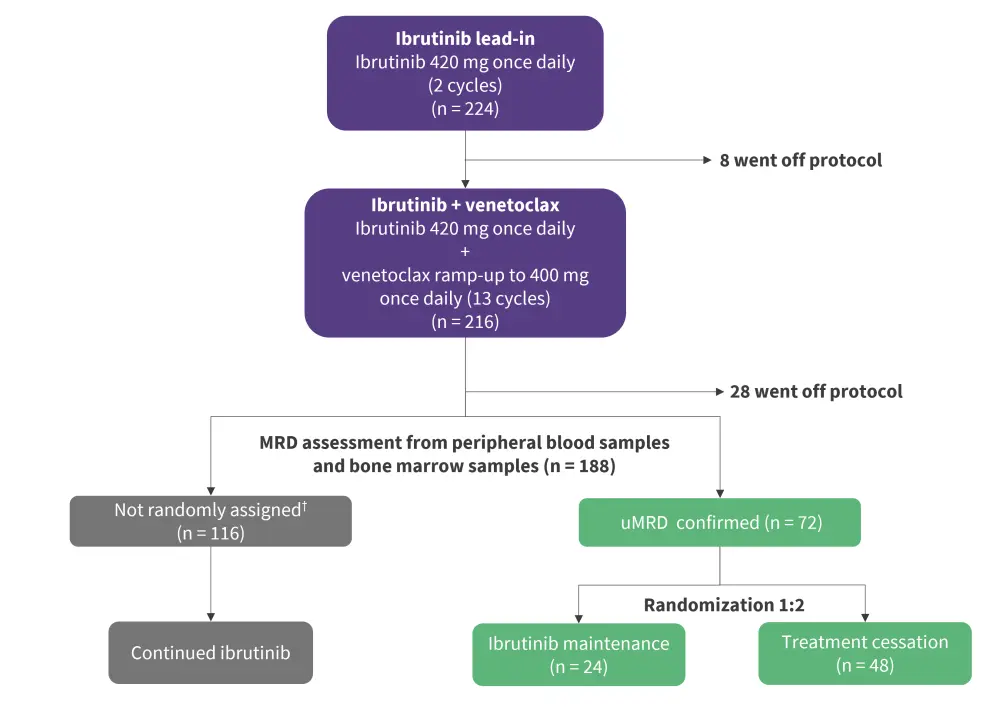
uMRD, undetectable minimal residual disease.
*Adapted from Kater et al.1
†Patients not randomly assigned included 110 patients who were MRD positive in peripheral blood or bone marrow, four patients who were enrolled before protocol amendment, and two patients in error.
Results
Patient characteristics
Between July 12, 2017, and Jan 21, 2019, 230 patients were enrolled and 225 were eligible for treatment. The median age was 68 years and there were no significant differences in baseline characteristics between randomly assigned patients and patients who were not randomly assigned (uMRD vs MRD-positive or off protocol). Selected baseline characteristics are summarized in Table 1.
Table 1. Selected patient characteristics*
|
CLL, chronic lymphocytic leukemia; IGHV, immunoglobulin heavy chain variable region; IQR, interquartile range. |
||||
|
Characteristics, % |
Ibrutinib |
Treatment |
Patients not |
All patients |
|---|---|---|---|---|
|
Median age (IQR), |
66 (58–72) |
71 (64–73) |
68 (61–72) |
68 (61–72) |
|
Binet stage C |
38 |
46 |
44 |
44 |
|
Creatinine clearance |
73 (62–85) |
71 (59–86) |
70 (61–87) |
71 (60–87) |
|
TP53 mutation |
25 |
17 |
25 |
23 |
|
11q deletion |
29 |
38 |
27 |
30 |
|
17p13 deletion |
13 |
10 |
16 |
15 |
|
Trisomy 12 |
17 |
10 |
8 |
10 |
|
TP53 pathway |
25 |
19 |
26 |
24 |
|
Genomic complexity |
34 |
33 |
23 |
26 |
|
Unmutated IGHV |
58 |
67 |
64 |
64 |
|
Previous lines of |
1 (1–3; 1–2) |
1 (1–8; 1–2) |
1 (1–15; 1–2) |
1 (1–15; 1–2) |
Clinical outcomes
Planned treatment with ibrutinib plus venetoclax, until random assignment at Cycle 15, was completed by 84% of patients. At Cycle 15, 36% of patients in the intention-to-treat population had uMRD in both peripheral blood and bone marrow (Figure 2).
Figure 1. MRD rates and response *
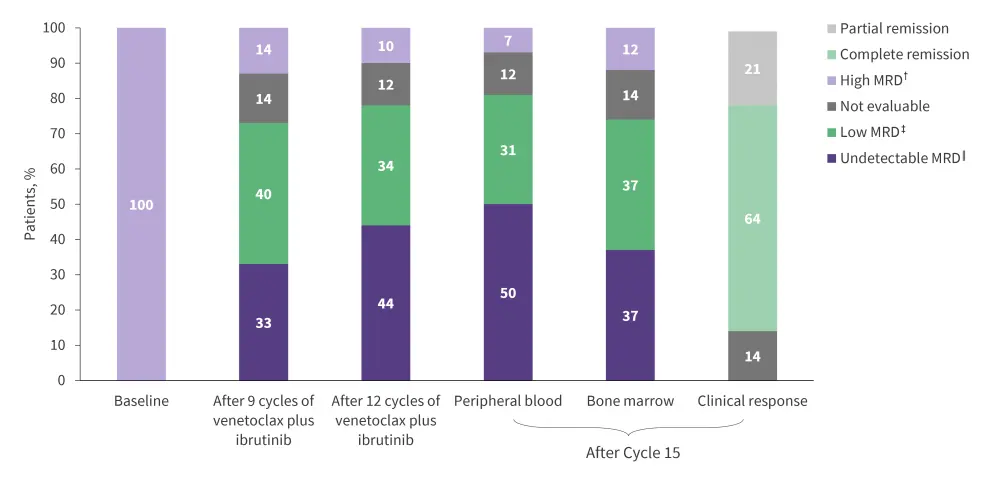
MRD, minimal residual disease.
*Adapted from Kater, et al.1
†High MRD = ≥10−2.
‡Low MRD = ≥10−4 and <10−2.
‖Undetectable MRD = <10−4.
After a median follow-up of 34.4 months, the primary endpoint was met with an estimated 27-month PFS of 98% (95% confidence interval [CI], 89–100) for patients with uMRD in the treatment cessation group. For patients in the ibrutinib maintenance group and those not randomly assigned who continued Ibrutinib after Cycle 15, the estimated 27-month PFS was 96% (95% CI, 79–100) and 97% (95% CI, 93–99), respectively. There was no difference in uMRD in peripheral blood at Cycle 15 between patients with or without high-risk features: TP53 aberrations, immunoglobulin heavy chain gene mutational status, and genomic complexity.
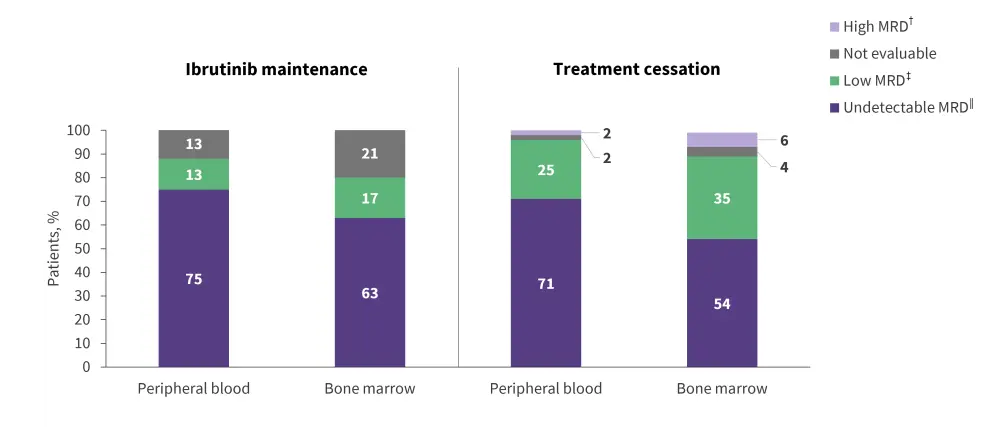
Of patients with uMRD after Cycle 15, MRD at 27 months was similar between the ibrutinib maintenance and treatment cessation groups (Figure 3). In the treatment cessation group, seven patients reinitiated therapy due to MRD positivity or clinical relapse, and six patients who were evaluated showed clinical remission.
Figure 1. MRD rates in peripheral blood and bone marrow aspirates 27 months after treatment for patients in the ibrutinib maintenance group or treatment cessation group*
footer
MRD, minimal residual disease.
*Adapted from Kater, et al.1
†High MRD = ≥10−2.
‡Low MRD = ≥10−4 and <10−2.
‖Undetectable MRD = <10−4.
The estimated 27-month overall survival was 94% (95% CI, 90–97) for all eligible patients, 100% (95% CI, 86–100) for the ibrutinib maintenance group, 98% (95% CI, 89–100) for the treatment cessation group, and 92% (95% CI, 86–95) for patients not randomly assigned.
- Five patients died during Cycles 1–2 of ibrutinib monotherapy
- Six patients died during combination therapy with ibrutinib plus venetoclax up to Cycle 15
- One patient died during observation in the treatment cessation group
- Two patients died during ibrutinib maintenance among the patients not randomly assigned
Treatment-emergent adverse events (TEAEs) are summarized in Table 2.
Table 2. Summary of ≥Grade 3 TEAEs after Cycle 15*
|
TEAE, treatment-emergent adverse event. |
|||
|
≥Grade 3 TEAE, % |
Ibrutinib continuation |
Treatment cessation |
Patients not |
|---|---|---|---|
|
Any |
38 |
15 |
41 |
|
Infections |
21 |
4 |
15 |
|
Neutropenia |
0 |
4 |
3 |
|
Bleeding |
4 |
0 |
0 |
|
Malignancies, neoplasm |
8 |
2 |
6 |
|
Hypertension |
4 |
0 |
2 |
Conclusion
The primary endpoint of this study was met, with a 98% PFS rate at 27 months for patients with uMRD who stopped treatment with ibrutinib plus venetoclax. TP53 aberrations, complex karyotype, and unmutated immunoglobulin heavy chain variable region did not affect the rate of uMRD. These results suggest a favorable benefit-risk profile for MRD-guided ibrutinib plus venetoclax treatment cessation and reinitiation in patients with R/R CLL; however, long-term survival data remains to be seen. No patients progressed after treatment cessation and those who became MRD positive during observation successfully reinitiated therapy with the same regimen. This demonstrates the potential of MRD-guided cessation and reinitiation of targeted therapy for patients with R/R CLL in the first-line treatment setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


