All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Novel combinations for the frontline treatment of patients with DLBCL
The combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) is currently the standard of care for treatment-naïve patients with diffuse large B-cell lymphoma (DLBCL). However, over one-third of patients are not cured by R-CHOP treatment. Thus, new frontline treatment options are needed for patients with DLBCL, particularly for those who have inferior outcomes with R-CHOP.
Below, we summarize key data from a recent article published in Journal of Clinical Oncology and presentations from the 64th American Society of Hematology (ASH) Annual Meeting and Exposition on novel frontline treatment combinations in DLBCL. Davies et al. highlighted improved outcomes in DLBCL molecular subgroups with the addition of bortezomib to R-CHOP chemoimmunotherapy, based on 5-year survival results from the REMoDL-B trial1, 2; while Topp et al. presented the favorable response rates and safety profile of glofitamab plus R-CHOP in patients with previously untreated DLBCL, from a phase Ib study.3
Bortezomib + R-CHOP in patients with DLBCL: 5-year survival results from the REMoDL-B Trial1
REMoDL-B (NCT01324596) was an open-label, randomized, phase III trial investigating frontline R-CHOP + bortezomib (RB-CHOP) chemoimmunotherapy in patients with DLBCL. The study was conducted between June 2011 and June 2015 across 107 sites in the UK and Switzerland. Eligible patients were randomized to receive R-CHOP or RB-CHOP from Cycle 2 and stratified by cell-of-origin as per gene expression profile analysis.
The primary endpoints were progression-free survival (PFS) in R-CHOP vs RB-CHOP study arms and evaluation of DLBCL molecular phenotype (ABC/MHG/GCB) effect on potential benefit derived from the addition of bortezomib. Secondary endpoints included overall survival (OS), toxicity, and quality of life.
Results
Efficacy
At a median follow-up of 60 months, there was no overall difference in PFS and OS between R-CHOP and RB-CHOP groups;
- 5-year PFS of 64% and 70%, respectively (p = 0.085); and
- 5-year OS of 76% vs 79%, respectively (p = 0.32).
The addition of bortezomib improved PFS and OS for RB-CHOP treated patients in the ABC and MHG subgroups (Figure 1), but not in the GCB subgroup (data not shown). In summary, 5-year PFS improved by 15% and 26% in the ABC and MHG subgroups, respectively, and a 13% improvement in 5-year OS was observed in the ABC subgroup.
There were no differences in outcomes by treatment arm in any of the subgroups by LymphGen classification.
Figure 1. Five-year survival outcomes in the ABC and MHG subgroups*
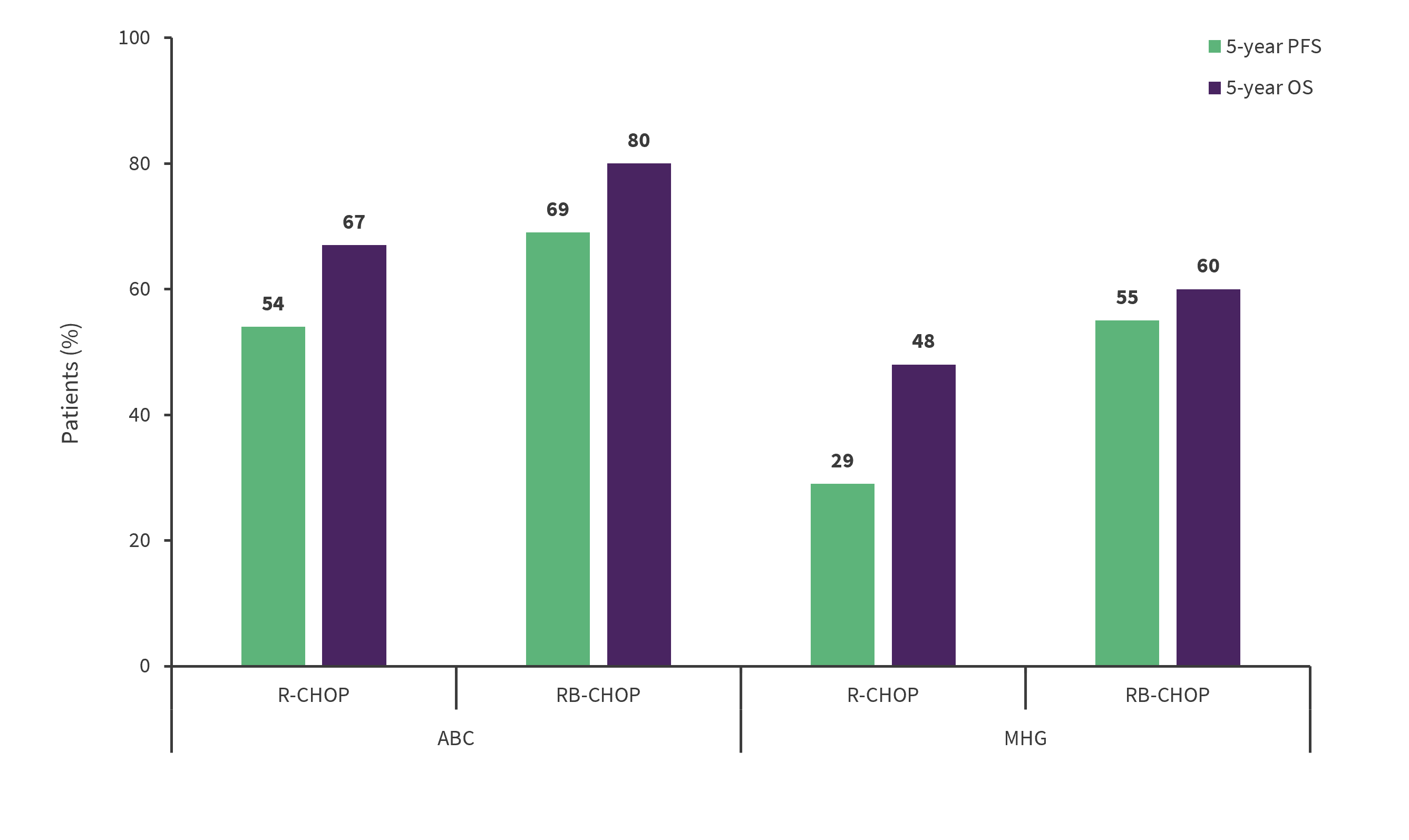
PFS, progression-free survival; OS, overall survival; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; RB-CHOP, R-CHOP + bortezomib.
*Adapted from Davies, et al.1
Safety
The addition of bortezomib was well tolerated and 5-year follow-up safety data was similar to the preliminary analysis of the trial at a median 30-month follow-up; no new adverse events (AEs) were reported. More serious AEs were reported in the RB-CHOP group vs the R-CHOP group (50.7% and 42.5%, respectively). There were four and five treatment-related deaths in the BR-CHOP group and R-CHOP group, respectively. BR-CHOP was not associated with increased hematological toxicity.
Author’s conclusions
The addition of bortezomib to frontline R-CHOP treatment improved survival outcomes in patients with DLBCL, specifically in ABC and MHG subtypes. Gene expression profiling can help identify molecular subtypes of DLBCL that may benefit from the addition of bortezomib to initial therapy.
Glofitamab + R-CHOP in patients with previously untreated DLBCL: Phase Ib study results2
Study design
This was a phase Ib, multi-center study of glofitamab + R-CHOP (G/R-CHOP) in patients with DLBCL (NCT03467373). A total of six to eight 21-day cycles of R-CHOP were administered to patients included in the study. In Cycle (C) 1, R-CHOP was given followed by intravenous glofitamab (2.5/10/30 mg) during C2 (Day [D] 8, 2.5 mg; D15, 10 mg), with the target dose (30 mg) given from C3D8 onwards. Hospitalization was at the discretion of the investigator for patients enrolled in the expansion stage.
The study aimed to assess the efficacy and safety of G/R-CHOP from the run-in portion and expansion stage of the study.
Results
Overall, 56 patients received R-CHOP (safety population) and 53/56 patients received glofitamab in addition to R-CHOP. At baseline, the majority of patients had a high tumor burden.
Efficacy
After a median 5.6-month follow-up, promising response rates were observed with G/R-CHOP in the end of treatment population (Table 1).
Table 1. Response rates*
|
CMR, complete molecular response; EOT, end of treatment; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; G/R-CHOP, glofitamab+R-CHOP. |
|||
|
Efficacy endpoint, % |
R-CHOP |
G/R-CHOP |
|
|---|---|---|---|
|
EOT response |
|||
|
CMR rate |
72.7 |
75.5 |
|
|
Overall response rate |
83.6 |
86.8 |
|
|
Best overall response§ |
|||
|
CMR rate |
72.7 |
75.5 |
|
|
Overall response rate |
92.7 |
96.2 |
|
G/R-CHOP frontline treatment showed overall promising and consistent antitumor activity in treated patients.
Safety
- Safety results are summarized in Table 2. Cytokine release syndrome events were all low grade (Grade 1/2), manageable, and resolved.
- The most common AEs (≥15%) were neutropenia, anemia, nausea, diarrhea, thrombocytopenia, constipation, infusion-related reactions, and COVID-19 pneumonia. No Grade 5 AEs were related to glofitamab.
The hospitalization requirement was removed following the safety run-in and the subsequent 42 patients were treated without mandatory hospitalization.
Table 2. Safety profile*
|
AE, adverse event; CRS, cytokine release syndrome; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; G/R-CHOP, glofitamab+R-CHOP. |
|
|
Parameter, n |
Safety population |
|---|---|
|
Total AEs |
55 |
|
Related to glofitamab |
32 |
|
Grade 3/4 AE |
36 |
|
Related to glofitamab |
13 |
|
Grade 5 AE† |
4 |
|
Related to glofitamab |
0 |
|
Serious AE |
18 |
|
Related to glofitamab |
5 |
|
AE leading to discontinuation of glofitamab‡ |
1 |
|
AE leading to dose modification/interruption of glofitamab |
12 |
|
CRS (any grade) |
6 |
|
Grade 1 |
4 |
|
Grade 2 |
2 |
|
Grade ≥3 |
0 |
|
Serious AE of CRS (any grade) |
2 |
|
Median time to 1st CRS event, hours§ |
10.2 |
|
Tocilizumab for CRS management‖ |
2/6 |
|
CRS resolved |
6/6 |
|
Other AEs of interest |
|
|
Neurological AEs¶ |
22 |
|
Grade 1 |
16 |
|
Grade 2 |
4 |
|
Grade 3# |
2 |
|
Infections |
22 |
|
Serious infections |
9 |
|
COVID-19 |
8 |
|
Sepsis |
1 |
|
Neutropenia |
25 |
|
Grade ≥3 |
23 |
|
Febrile neutropenia** |
7 |
Author’s conclusions
The presented data suggest that G/R-CHOP can be safely used in combination as a frontline fixed-duration treatment for patients with DLBCL considering the low incidence and severity of cytokine release syndrome, minimal toxicity, and promising efficacy reported. All patients received R-CHOP at the same dose intensity and the use of GR-CHOP in an outpatient setting may also be appropriate.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

