All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Parsaclisib, a potent and highly selective next-generation PI3Kδ inhibitor: a summary of data from the three CITADEL trials (203, 204, and 205)
Non-Hodgkin lymphoma (NHL) includes a variety of subtypes and the behavior, treatment, and outcomes associated with NHL are equally varied. Most NHLs, however, originate from B cells, and B-cell NHL may be further categorized into its indolent (follicular lymphoma [FL] and marginal zone lymphoma [MZL]) and aggressive (diffuse large B-cell lymphoma [DLBCL] and mantle cell lymphoma [MCL]) forms. While outcomes have improved for both the indolent and aggressive NHLs, relapse is still a common occurrence, and relapsed disease is associated with poorer outcomes.1
Phosphatidylinositol 3-kinase (PI3K) inhibitors have emerged as potential treatment options, with four of these agents (idelalisib, copanlisib, duvelisib, and umbralisib) currently approved for various indications. There are several PI3K isoforms (α, β, γ, and δ) that may be targeted by these agents, and each of these isoforms plays one or more roles in both B-cell NHLs and in healthy lymphocytes, which has led to challenges associated with the safety of PI3K inhibitors.1
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, three abstracts were presented regarding parsaclisib, a potent and highly selective next-generation PI3Kδ inhibitor, in the treatment of relapsed or refractory (R/R) FL (CITADEL-203, presented by Ryan C. Lynch), R/R MZL (CITADEL-204, presented by Tycel Phillips), and R/R MCL (CITADEL-205, presented by Amitkumar Mehta), respectively. We summarize the data presented below.2-4
CITADEL-203: Lynch, et al. (Follicular lymphoma)
Study design
CITADEL-203 is a phase 2, multicenter, open-label trial of parsaclisib monotherapy in patients with R/R FL.2 Eligible patients:
- were ≥18 years of age;
- had histologically confirmed Grade 1, 2 or 3a R/R FL;
- had received at least two prior systemic therapies (not including PI3K or Bruton’s tyrosine kinase [BTK] inhibitors];
- had an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) score ≤2; and
- were ineligible for hematopoietic stem cell transplantation (HSCT).
The primary endpoint was objective response rate, and secondary endpoints included complete response (CR) rate, duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety.
Patients received 20 mg parsaclisib once daily for 8 weeks, at which point patients in the weekly dosing group (WG) received 20 mg parsaclisib once weekly, and patients in the daily dosing group (DG) received 2.5 mg parsaclisib daily (Figure 1). All patients received prophylaxis for Pneumocystis jirovecii pneumonia. Daily dosing was selected during an interim analysis as the recommended dosing regimen.
Figure 1. CITADEL-203 study design*
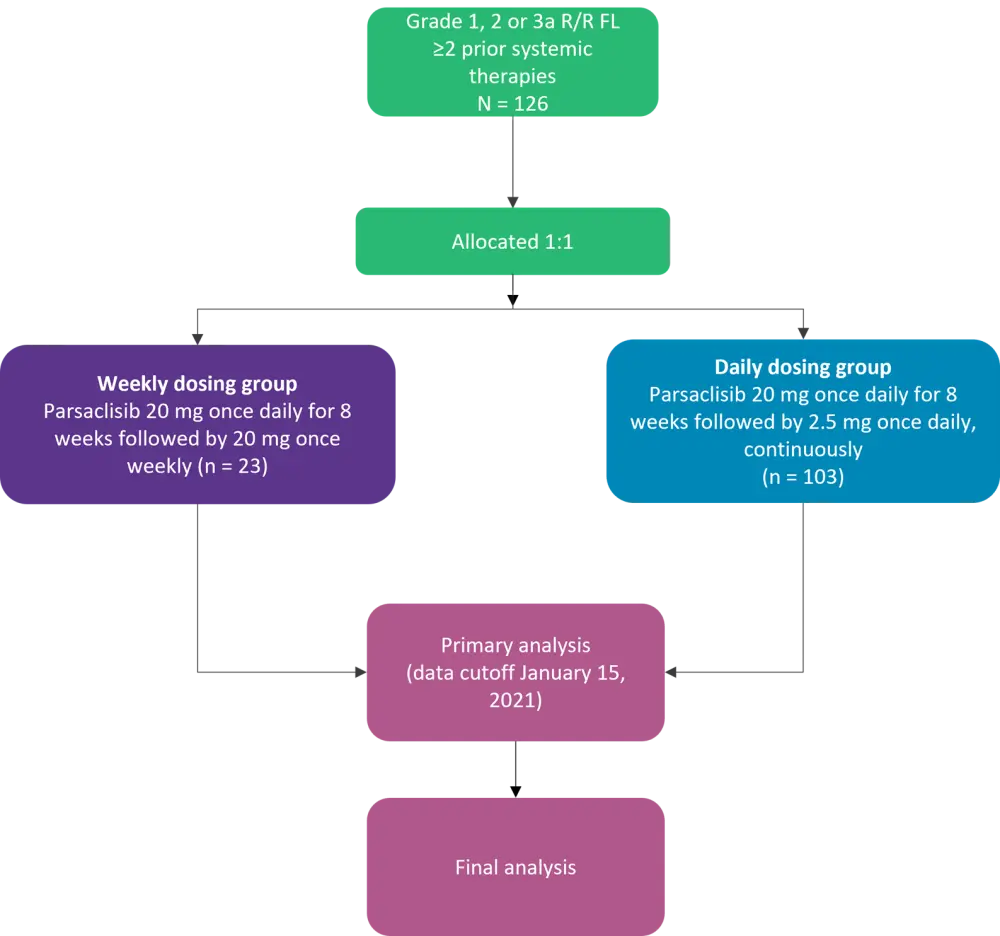
FL, follicular lymphoma; R/R, relapsed/refractory.
*Adapted from Lynch, et al.2
Baseline characteristics
The data cutoff for the primary analysis was January 15, 2021, at which point 126 patients had been treated (23 in the WG and 103 in the DG). The median age was 67.5 years (range, 40‒88 years), and 55.6% were male.
- The median time since initial diagnosis was 5.95 years (range, 0.2‒32.2 years).
- Most patients (93.7%) had an ECOG PS ≤1.
- The median number of prior lines of therapy was 2 (range, 1‒8); 27.8% of patients had received 3 prior lines of systemic therapy.
- In total, 41.3% had relapsed disease and 49.2% were refractory to their most recent prior therapy.
Results
Efficacy
The median treatment duration was 8.5 months (range, 0.5–27.2 months) and the median follow up was 20.6 months (range, 5.7‒34.1 months) for all treated patients. In the DG, median treatment duration and median follow up were 8.4 months (range, 0.8‒27.2 months) and 17.6 months (range, 5.7‒33.1 months), respectively.
Responses in the overall cohort and the weekly and daily dosing groups are shown in Table 1.
Table 1. Responses by dosing group and overall (per independent review committee)*
|
CI, confidence interval; DG, daily dosing group; ORR, objective response rate; WG, weekly dosing group. |
|||
|
Response, % |
WG (n = 23) |
DG (n = 103) |
Total (N = 126) |
|---|---|---|---|
|
ORR |
65.2 |
77.7 |
75.4 |
|
95% CI |
42.7‒83.6 |
68.4‒85.3 |
66.9‒82.6 |
|
Complete response |
13.0 |
19.4 |
18.3 |
|
Partial response |
52.2 |
58.3 |
57.1 |
- Among all patients with a CR or partial response (PR), 73.7% of responses occurred at the first disease assessment.
- The median DoR was 14.7 months for the entire cohort (range, 11.3‒19.6 months) and the DG (10.4‒not estimable [NE] months).
- Median PFS was 14.0 months for the entire cohort (range, 11.3‒19.6 months) and 15.8 months for the DG (11.0‒NE months).
- Median OS had not been reached at the time of data cutoff.
Safety
Treatment-emergent adverse events (TEAEs) occurred in almost all patients (97.6%), and Grade 3 or higher TEAEs occurred in 58.7% of patients.
- The most common TEAEs were diarrhea (38.1%), nausea (24.6%), and cough (22.2%).
- The most common Grade ≥3 TEAEs were diarrhea (11.9%) and neutropenia (10.3%).
- Overall, 46.8% of patients had TEAEs leading to dose interruption and 17.5% of patients had TEAEs leading to dose reduction.
- TEAEs leading to treatment discontinuation occurred in 23.8% of patients.
- The most common TEAEs leading to treatment discontinuation were diarrhea (7.1%), colitis (4.0%), pneumonitis (2.4%), and rash (2.4%).
- In total, 45.2% of patients experienced serious TEAEs, the most common of which were diarrhea (7.1%), colitis (6.3%), and pneumonitis (2.4%).
- Two fatal AEs occurred (one due to Stevens-Johnson syndrome, one due to pneumonia) that were considered to be related to parsaclisib.
CITADEL-204: Phillips, et al. (Marginal zone lymphoma)
Study design
CITADEL-204 is a phase 2, open-label study of parsaclisib as monotherapy for the treatment of patients with R/R MZL.3 Eligible patients:
- were ≥18 years of age;
- had histologically confirmed MZL;
- had received at least one prior line of systemic therapy (including anti-CD20 therapy);
- had disease progression or inadequate response to their most recent line of treatment;
- had ECOG PS ≤2; and
- had measurable lymphadenopathy (nodal MZL), lymphoid malignancy (extranodal MZL) or histologically confirmed bone marrow (BM) infiltration (splenic MZL).
The primary and secondary endpoints and treatment allocation/dosing (WG and DG) were the same as CITADEL-203. Patients in CITADEL-204 were also treated prophylactically for P. jirovecii pneumonia.
Baseline characteristics
Two cohorts were enrolled in this study. Cohort 1 included patients who had been treated previously with ibrutinib (n = 10) and Cohort 2 included patients who were naïve to BTK inhibitor therapy (n = 100). This abstract presentation included data from Cohort 2 only. Twenty-eight patients in Cohort 2 were assigned to the WG, and 72 were assigned to the DG. The median age was 71.0 years (range, 35‒95 years), and 53.0% were male.
- Of the 100 patients in Cohort 2, 31.0% had nodal MZL, 34.0% had extranodal MZL, and 35.0% had splenic MZL (95.0% had ECOG PS ≤1).
- The median number of prior lines of therapy was 2 (range, 1‒8 lines of therapy); 20.0% had received at least 3 prior lines of systemic therapy.
- In total, 46.0% had relapsed disease, and 49.0% were refractory to their most recent line of therapy.
Results
Efficacy
The median treatment duration was 13.4 months (range, 0.4‒30.9 months), and the median follow up was 22.8 months (range, 11.9‒37.0 months) for all treated patients; in the DG, median treatment duration and median follow up were 11.6 months (range, 0.4‒30.9 months) and 21.0 months (range, 11.9‒37.0 months), respectively.
Responses in the weekly and daily dosing groups and by MZL subtypes in Cohort 2 are shown in Table 2.
Table 2. Responses in BTKi-naïve patients overall, by dosing group, and by MZL subtype*
|
CI, confidence interval; DG, daily dosing group; MZL, marginal zone lymphoma; ORR, objective response rate; WG, weekly dosing group. |
|||
|
By dosing group |
|||
|---|---|---|---|
|
Response, % |
WG (n = 28) |
DG (n = 72) |
Total (N = 100) |
|
ORR |
57.1 |
58.3 |
58.0 |
|
95% CI |
37.2‒75.5 |
46.1‒69.8 |
47.7‒67.8 |
|
Complete response |
10.7 |
4.2 |
6.0 |
|
Partial response |
46.4 |
54.2 |
52.0 |
|
By MZL subtype (both dosing groups) |
|||
|
Response, % |
Nodal (n = 31) |
Extranodal (n = 34) |
Splenic (n = 35) |
|
ORR |
51.6 |
55.9 |
65.7 |
|
95% CI |
33.1‒69.8 |
37.9‒72.8 |
47.8‒80.9 |
|
Complete response |
6.5 |
8.8 |
2.9 |
|
Partial response |
45.2 |
47.1 |
62.9 |
- Among all patients who achieved CR or PR, 65.5% of responses occurred at the first disease assessment, with a median time to response of 8.1 weeks.
- Median DoR was 12.2 months (range, 8.1‒17.5 months) for all patients and for those in the DG.
- Median PFS was 16.5 months for all patients (range, 13.5‒19.6 months) and for the DG (range, 11.5‒20.6 months).
- As of the data cutoff, median OS had not been reached.
Safety
TEAEs occurred in 96.0% of patients, and Grade ≥3 TEAEs occurred in 63.0% of patients.
- The most common TEAEs were diarrhea (47.0%) and cough (23.0%).
- The most common Grade ≥3 TEAEs were diarrhea (12.0%), neutropenia (9.0%), pneumonia (9.0%), and colitis (7.0%).
- TEAEs led to dose interruption, reduction, and discontinuation in 56.0%, 16.0%, and 29.0% of patients, respectively.
- The most common TEAEs leading to discontinuation were diarrhea (9.0%) and colitis (5.0%).
- Serious TEAEs occurred in 47.0% of patients; the most common of which was pneumonia (9.0%).
- Fatal TEAEs occurred in two patients: one death was due to febrile neutropenia, and one was due to sepsis; both were considered treatment related.
CITADEL-205: Mehta, et al. (Mantle cell lymphoma)
Study design
CITADEL-205 is a phase 2, open-label study investigating parsaclisib as monotherapy for patients with R/R MCL. Eligible patients:4
- were ≥18 years of age;
- had pathologically confirmed MCL with documented cyclin D1 overexpression or t(11;14);
- had ECOG PS ≤2; and
- received 1‒3 prior systemic therapies but had not had any prior treatment with a BTK and/or PI3K inhibitor.
The primary endpoint, as in the other two CITADEL trials, was ORR, and secondary endpoints were CRR, DoR, OS, PFS, and safety. Treatment and dosing were also the same as in the other two trials, with patients allocated into WG or DG after 8 weeks of daily parsaclisib treatment.
Baseline characteristics
As of the data cutoff, 108 patients who were naïve to BTK inhibitors had been treated, with 31 in the WG and 77 in the DG. The median age was 72 years (range, 43‒90 years), and 79.6% were male.
- Overall, 92.6% of patients had ECOG PS ≤1, and the median time since initial diagnosis was 3.6 years (range, 0.1‒20.9 years).
- The median number of prior lines of systemic therapy was 1 (range, 1‒3 lines of therapy), with 25.9% having received two prior lines of therapy.
- In total, 31.5% had received a prior HSCT, 50.0% had relapsed, and 43.5% were refractory to the most recent prior therapy.
Results
Efficacy
The median treatment duration was 8.3 months (range, 0.1‒30.0 months), and the median follow up was 22.9 months (range, 11.6‒35.9 months) for all treated patients; in the DG, median treatment duration and median follow up were 7.9 months (range, 1.7‒27.4 months) and 18.2 months (range, 11.6‒35.9 months), respectively.
Responses by treatment group and overall are shown in Table 3.
Table 3. Response by treatment group and overall*
|
CI, confidence interval; DG, daily dosing group; ORR, overall response rate; WG, weekly dosing group. |
|||
|
Response, % |
WG (n = 31) |
DG (n = 77) |
Total (N = 108) |
|---|---|---|---|
|
ORR |
64.5 |
70.1 |
68.5 |
|
95% CI |
45.4–80.8 |
58.6–80.0 |
58.9–77.1 |
|
Complete response |
22.6 |
15.6 |
17.6 |
|
Partial response |
41.9 |
54.5 |
50.9 |
- Among patients who achieved CR or PR, 89.2% of responses occurred at the first disease assessment.
- Median DoR was 13.7 months (range, 9.0‒19.9 months) for all patients and 12.1 months (range, 9.0-NE months) for the DG.
- Median PFS was 11.9 months (range, 8.3‒16.9 months) for all patients and 13.6 months (range, 10.0‒16.9 months) for the DG.
- Median OS had not been reached by the time of data cutoff.
Safety
TEAEs occurred in 90.7% of patients, with Grade ≥3 TEAEs in 62.0%.
- The most common TEAEs were diarrhea (34.3%), pyrexia (17.6%), and constipation (13.0%).
- The most common Grade ≥3 TEAEs were diarrhea (13.9%) and neutropenia (8.3%).
- TEAEs leading to dose interruption, reduction, and treatment discontinuation occurred in 47.2%, 8.3%, and 25.0% of patients, respectively.
- The most common TEAEs leading to treatment discontinuation were diarrhea (11.1%), colitis (4.6%), and hypokalemia (2.8%).
- Serious TEAEs occurred in 42.6%, the most common of which were diarrhea (9.3%) and colitis (4.6%).
- Six fatal TEAEs occurred, one of which was attributed to parsaclisib (a patient with leukocytosis, acute myelomonocytic leukemia, and acute kidney injury).
Conclusion
These three studies (CITADEL-203, CITADEL-204, and CITADEL-205) demonstrate rapid responses with parsaclisib in patients with FL, MZL, and MCL, respectively, and the responses seen have thus far been durable, with the caveat that the median follow up in these trials (at the point of data cutoff) was under 2 years. The safety data are largely in line with other PI3K/PI3Kδ inhibitors, with diarrhea and colitis consistently being the most common reason for discontinuation of parsaclisib. It will be interesting to see where parsaclisib falls among other PI3K inhibitors at the final analysis when efficacy data have matured, particularly OS data.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

