All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Phase I data of golidocitinib for R/R peripheral T-cell lymphoma: JACKPOT8 trial
Peripheral T-cell lymphomas (PTCL) are a group of heterogeneous non-Hodgkin lymphomas originating from mature T-cells. Patients with PTCL have a poor prognosis, particularly those with disease that is refractory to first-line therapy. There is a lack of consensus on standard treatment protocols and most currently approved therapies have shown limited effectiveness. Emerging data indicates that the JAK-STAT pathway may mediate pathogenesis of T-cell neoplasms; therefore, this is a potential target for therapeutic intervention. Golidocitinib, a novel JAK1 selective inhibitor, has demonstrated >200-fold selectivity over JAK2, JAK3, and TYK2.1 It is orally available and has favorable pharmacokinetic properties; maintaining efficacious blood concentration levels with a once daily dosing regimen.
Below, we report results from phase I of the JACKPOT 8 study (NCT04105010), a phase I/II study of golidocitinib in relapsed/refractory (R/R) PTCL, presented by Won-Seog Kim at the European Hematology Association (EHA) 2022 Congress.2
Study design
This is an ongoing multinational, single-arm, non-randomized, open-label, phase I/II study to determine the safety, tolerability, pharmacokinetics, and antitumor efficacy of golidocitinib in patients with R/R PTCL. The study involves dose escalation in phase I (n = 51) and dose expansion in phase II. In the dose escalation phase, R/R PTCL patients received golidocitinib at a dose of either 150 mg/day (n = 35) or 250 mg/day (n = 16) to determine the recommended phase II dose.
As of May 31, 2021, the JACKPOT8 cohort included 51 patients with R/R PTCL with a median age of 61 years (range, 29–79 years) and a median of 2 prior systemic therapies (range, 1–8 prior therapies). Ten patients (19.6%) had undergone hematopoietic stem cell transplantation and 15 patients (29.4%) had bone marrow involvement at baseline; the cohort also included a number of histologically confirmed PTCL subtypes (Figure 1). All patients had measurable disease on computer tomography, according to Lugano classification, and an Eastern Cooperative Oncology Group Performance Status of ≤2.
The primary endpoint was the adverse events associated with golidocitinib in patients with R/R PTCL, as determined by posttreatment Lugano assessment. Secondary endpoints were investigator-assessed overall response rate, duration of response (DOR), and progression-free survival.
Figure 1. Histologically confirmed PTCL subtypes*
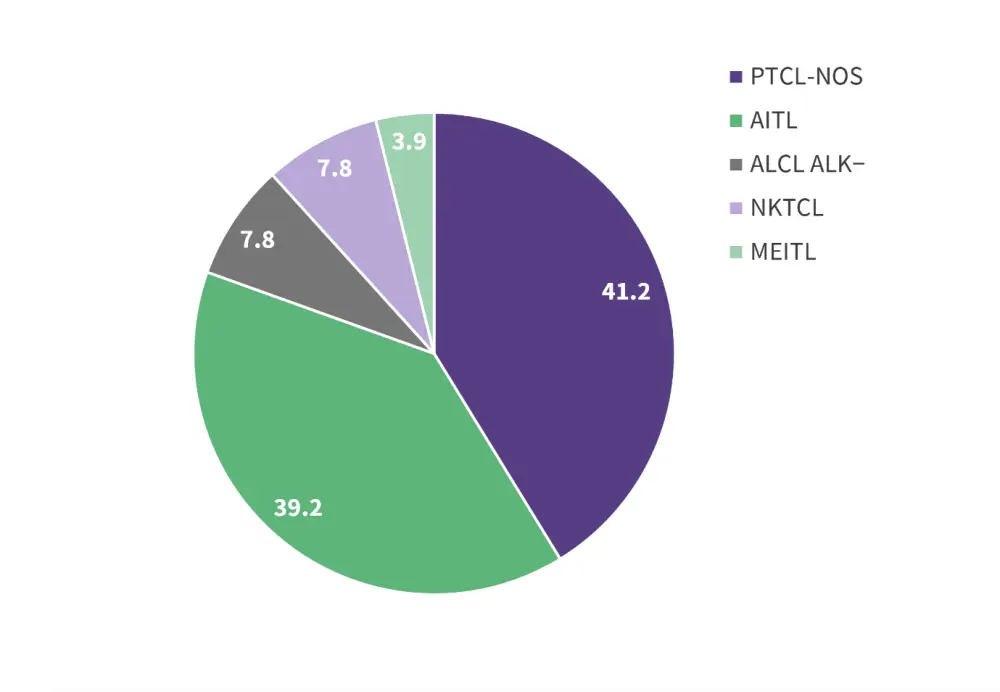
AITL, angioimmunoblastic T-cell lymphoma; ALCL ALK-, anaplastic lymphoma kinase-negative anaplastic large cell lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; NKTCL, extranodal-nasal/natural killer T-cell lymphoma; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma.
Results
At the data cut-off, 49 patients had completed at least one posttreatment Lugano assessment.
Efficacy2
Significant tumor responses were observed at both the 150 mg/day and 250 mg/day doses.
Figure 2. Response outcomes by dose group*
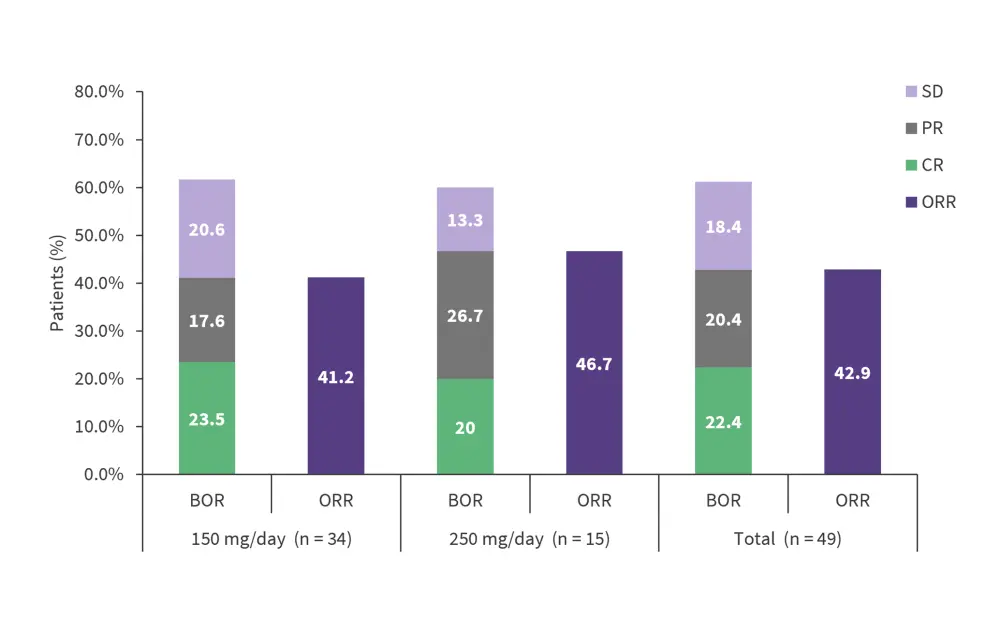
BOR, best overall response; CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease.
Efficacious tumor response was also observed across the various PTCL subtypes (Figure 3).
Figure 3. Efficacy outcomes by histological subtypes*
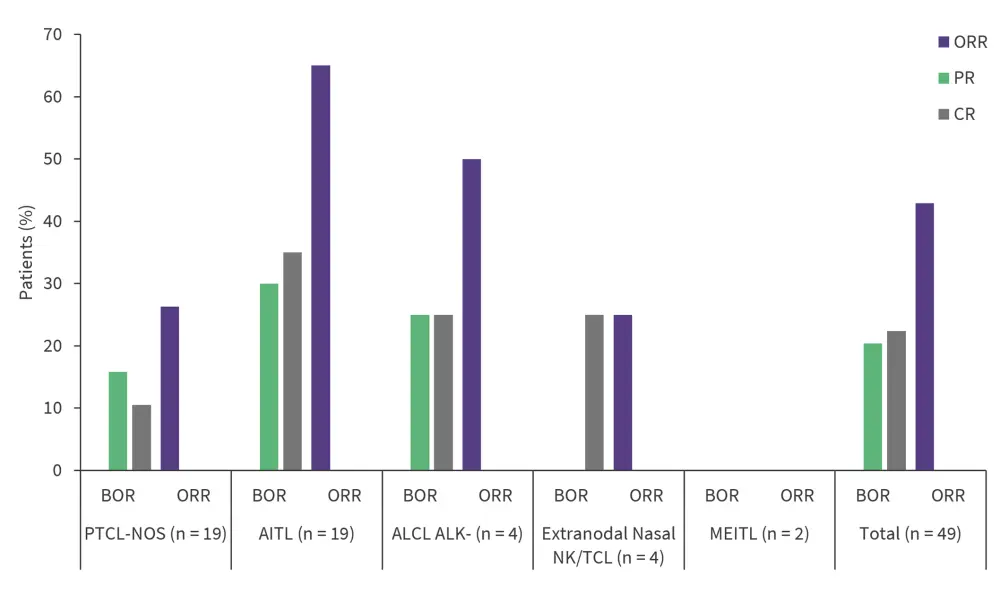
AITL, angioimmunoblastic T-cell lymphoma; ALCL ALK-, anaplastic lymphoma kinase-negative anaplastic large cell lymphoma; CR, complete response; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; NK/TCL, natural killer/T-cell lymphoma; NOS, not otherwise specified; ORR, overall response rate; PR, partial response; PTCL, peripheral T-cell lymphoma not otherwise specified.
At the data cut-off, median DOR had not been reached and the longest DOR was >14 months.
Safety2
- Overall, 48 patients (94.1%) experienced a treatment emergent adverse event (TEAE) of any grade, of which 30 (58.8%) experienced a ≥Grade 3 TEAE.
- Twenty patients (39.2%) experienced ≥Grade 3 TEAEs possibly related to the drug (as per investigator assessment).
- The most commonly experienced ≥Grade 3 TEAEs were:
- neutropenia (29.4%)
- thrombocytopenia (15.7%)
- pneumonia (11.8%)
- anemia (7.8%)
- hepatic enzyme increase (7.8%)
Conclusion
The study met its primary endpoint, elucidating the adverse events associated with golidocitinib in patients with R/R PTCL. Preliminary data has demonstrated a favorable safety profile, comparable with that of currently approved therapies. The majority of TEAEs were found to be reversable or clinically manageable with dose alterations. Golidocitinib also demonstrated promising antitumor activity across multiple histologically defined subtypes. The next stage of the trial, a phase II, single arm, open-label study of golidocitinib in R/R PTCL is currently ongoing, and is based on the 250 mg/day dose identified in phase I. In conclusion, golidocitinib has demonstrated promising efficacy and a tolerable safety profile in R/R PTCL patients, indicating it may serve as a novel therapeutic option for this patient group.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

