All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Phase I trial of the triplet therapy ipilimumab, nivolumab, and brentuximab vedotin for patients with relapsed/refractory Hodgkin lymphoma
The tumor microenvironment of classical Hodgkin lymphoma (cHL) is composed of Hodgkin Reed-Sternberg cells, which constitute < 1% of the tumor volume, and the stroma. These primary lymphoma tumor cells overexpress programmed cell death ligand 1 and 2 (PD-L1 and PD-L2), which facilitate evasion of immune surveillance and survival within the tumor microenvironment. Approximately 15% of patients with cHL have chromosomal rearrangements of CIITA, the master regulator of MHC class II expression, which result in its down regulation. There are also high numbers of CD4+ cells, with an expansion of regulatory T cells. Therapies targeting the tumor alone are inadequate to induce a high complete remission rate in patients with relapsed/refractory (R/R) cHL.1
Therefore, Catherine Diefenbach and colleagues investigated a novel approach of combining the use of the checkpoint inhibitors ipilimumab and nivolumab, to potentiate the peritumoral T cells, with the CD30-specific antibody-drug conjugate brentuximab vedotin, to target the Hodgkin Reed-Sternberg cells. Results of the phase I portion of this clinical trial (NCT01896999) were recently published in The Lancet Haematology.1
Study design/patient characteristics1
- An open label multicenter phase I/II trial in adult patients with R/R cHL
- A total of 64 adult patients who had relapsed after ≥ 1 line of therapy, were enrolled into the study between March 7, 2014, and December 28, 2017
- 61 patients were evaluable for analysis
- Patients were sequentially assigned to one of three treatment regimens
- Ipilimumab plus brentuximab vedotin (ipilimumab group)
- Nivolumab plus brentuximab vedotin (nivolumab group)
- Ipilimumab plus nivolumab plus brentuximab vedotin (triplet group)
- All cycles were 21 days long, and all treatments were administered intravenously (IV).
- All patients received premedication with 40 mg prophylactic famotidine IV and 50 mg diphenhydramine at every cycle
- After the fifth treatment cycle, if the patient had no infusion reactions diphenhydramine was lowered to 25 mg or omitted per investigator discretion.
- The ipilimumab group used a modified 3 × 3 design (6 + 6), while the nivolumab and triplet group used a standard 3 + 3 design for dose escalation, demonstrated in Figure 1.
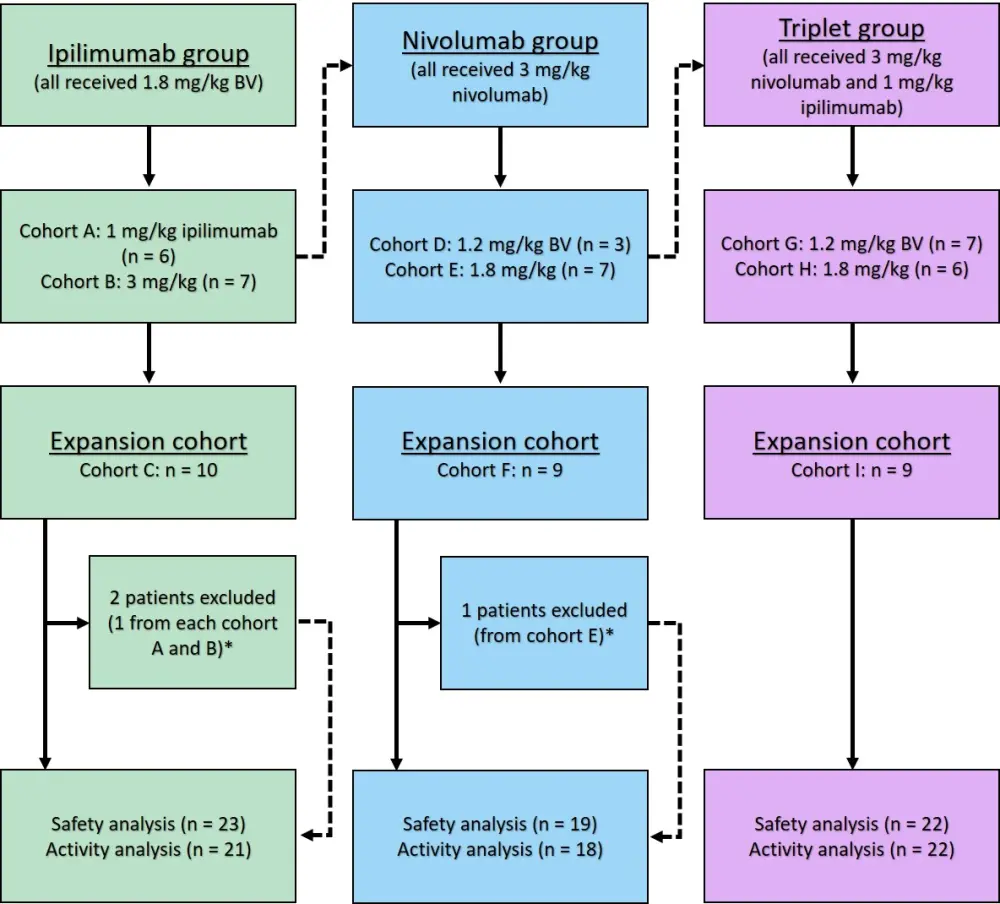
BV, brentuximab vedotin.
*Patients were excluded due to ineligibility.
- Nivolumab was administered on Day 1 of every cycle.
- Ipilimumab was administered on Day 1 of Cycle 1. Thereafter, ipilimumab was given every 6 weeks for up to 1 year in the ipilimumab group, and every 12 weeks for up to 2 years in the triplet group.
- Primary outcome measures: Maximum tolerated dose (MTD; highest dose with < 2 dose limiting toxicities observed out of six patients) and dose-limiting toxicity (DLT) of each combination.
- Secondary endpoints: complete response, overall response rate (ORR), duration of response, progression-free survival (PFS), overall survival.
- Baseline patient characteristics are summarized in Table 1.
Table 1. Baseline patient characteristics1
|
BV, brentuximab vedotin; ECOG, Eastern Cooperative Oncology Group; N/A, not available |
|||
|
Characteristic |
Ipilimumab group (n = 21) |
Nivolumab group (n =18) |
Triplet group (n = 22) |
|---|---|---|---|
|
Age, years (range) |
33 (30–40) |
40 (26–51) |
35 (27–40) |
|
Women/men, % |
48/52 |
50/50 |
50/50 |
|
Stage, % |
|
|
|
|
I |
0 |
6 |
9 |
|
II |
48 |
39 |
55 |
|
III |
29 |
22 |
14 |
|
IV |
24 |
33 |
23 |
|
ECOG performance status, % |
|
|
|
|
0 |
76 |
56 |
55 |
|
1–2 |
24 |
44 |
45 |
|
Number of extra nodal sites, % |
|
|
|
|
0–1 |
86 |
89 |
91 |
|
≥ 2 |
14 |
6 |
9 |
|
Bulky disease (≥ 7cm), % |
0 |
11 |
9 |
|
No. of previous therapies, % |
|
|
|
|
1 |
43 |
50 |
36 |
|
2 |
24 |
17 |
41 |
|
3 |
10 |
22 |
18 |
|
≥ 4 |
24 |
11 |
5 |
|
Previous BV |
14 |
22 |
5 |
|
Median time since last BV, years (range) |
1.20 (1.14–2.94) |
1.27 (0.85–1.78) |
1.34 (N/A) |
|
Response to last therapy, % |
|
|
|
|
Refractory |
48 |
50 |
73 |
|
≥ 6 months ≤ 1 year |
19 |
22 |
9 |
|
Response ≥ 1 year |
24 |
28 |
14 |
|
Unevaluable |
10 |
0 |
5 |
Results1
Dose limiting toxicities
Five DLTs were reported during dose escalation and one DLT was reported during dose expansion.
- One patient in the nivolumab group (Cohort D) experienced Grade 4 pneumonitis and Grade 3 typhlitis.
- During dose expansion one patient in the nivolumab group (Cohort F) experienced Grade 3 aminotransferase (aspartate aminotransferase and alanine aminotransferase) elevation.
- One patient in the triplet group (cohort E) experienced a Grade 4 diabetic ketoacidosis, although they had no previous history of diabetes.
- During dose expansion, one patient in the triplet group (Cohort I) experienced a post-allogeneic HSCT Grade 4 graft-versus-host disease or Stevens-Johnson syndrome.
Maximum tolerated dose
The MTDs of each therapy in the respective treatment groups are summarized in Table 2. Once the MTDs were established, patients were enrolled in the expansion cohorts with these schedules.
Table 2. Maximum tolerated dose of each therapy1
|
BV, brentuximab vedotin |
|||
|
Maximum tolerated dose |
Ipilimumab group |
Nivolumab group |
Triplet group |
|---|---|---|---|
|
BV dose, mg/kg |
1.8 |
1.8 |
1.8 |
|
Ipilimumab dose, mg/kg |
3.0 |
— |
1.0 |
|
Nivolumab dose, mg/kg |
— |
3.0 |
3.0 |
Toxicities
- The most common Grade 1–2 toxicities across all cohorts were peripheral sensory neuropathy (52%), fatigue (41%), diarrhea (41%), elevated alanine aminotransferase (34%) and aspartate aminotransferase (27%), and fever (25%).
- Grade ≥ 3 toxicities that occurred in greater than 5% of patients in at least one cohort are summarized in Table 3.
- The triplet group had the highest frequency of Grade ≥ 3 toxicities
- Pneumonitis only occurred in the nivolumab group (11%)
- Maculopapular rash occurred more frequently in the ipilimumab group (at 22%) than any other group
- Treatment related death due to pneumonitis occurred in one patient in the nivolumab group and one patient in the triplet group.
Table 3. Grade ≥ 3 toxicities1
|
WBC, white blood cell |
|||
|
Grade ≥ 3 toxicity in > 5% of patients |
Ipilimumab group (n = 23) |
Nivolumab group (n =19) |
Triplet group (n = 22) |
|---|---|---|---|
|
Fatigue, % |
0 |
0 |
9 |
|
Increased lipase, % |
0 |
0 |
9 |
|
Pneumonitis, % |
0 |
11 |
0 |
|
Maculopapular rash, % |
22 |
5 |
9 |
|
Vomiting, % |
4 |
0 |
9 |
|
Decreased WBC count, % |
0 |
0 |
9 |
- The median number of treatment cycles received were 7, 7, and 5.5 in the ipilimumab, nivolumab, and triplet groups, respectively.
- The most common reasons for treatment discontinuation in the ipilimumab, nivolumab, and triplet groups were
- treatment related adverse events (14%, 11%, and 32%, respectively)
- disease progression (29%, 11%, and 5%, respectively)
- alternative therapy (24%, 44%, and 36%, respectively).
- A total of 22 patients came off treatment and received autologous stem cell transplant.
Outcomes
- The median time to best response was 72 days for the ipilimumab group, and 66 days in the nivolumab and triplet groups.
- The median follow-up time for the ipilimumab, nivolumab, and triplet groups were 2.6, 2.4, and 1.7 years, respectively.
- Table 4 summarizes the response data.
- ORR rates were higher for the nivolumab (89%) and triplet (82%) groups compared with the ipilimumab group (76%).
Table 4. Response data1
|
CI, confidence interval; CR, complete response; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival. |
|||
|
Response |
Ipilimumab group |
Nivolumab group (n =18) |
Triplet group (n = 22) |
|---|---|---|---|
|
ORR, % (95% CI) |
76 (53–92) |
89 (65–99) |
82 (60–95) |
|
CR, % (95% CI) |
57 (34–78) |
61 (36–83) |
73 (50–89) |
|
1-year PFS, % (95% CI) |
61 (43–86) |
70 (50–96) |
80 (64–100) |
|
Median PFS, years (95% CI) |
1.2 (0.74–NR) |
NR |
NR |
|
Median OS |
NR |
NR |
NR |
Conclusion
The combination of brentuximab vedotin with ipilimumab, nivolumab, or both, was safe and well tolerated in adult patients with R/R cHL. However, the incidence of toxicities was higher in the triplet regimen, raising a possible concern for potentially higher immune activation related toxicity. Although the study may have been limited by the heterogeneity of the patient population, such as the number of previous treatments, and a small sample size, each treatment regimen also had a complete remission rate significantly higher than expected for the individual monotherapies. The nivolumab and triplet group had a higher response rate and better PFS than the ipilimumab regimen. Therefore, going forward, the phase II portion of the study will evaluate the tolerability and activity of nivolumab and brentuximab vedotin, and the triplet therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

