All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Polatuzumab vedotin + R-CHP vs. R-CHOP for frontline treatment of DLBCL: Results from the phase III POLARIX trial
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Hervé Tilly kicked off the late-breaking abstracts session with a presentation of data from POLARIX, a phase III trial investigating polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) versus standard of care (R-CHOP, which consists of the above named drugs in R-CHP with the addition of vincristine) as frontline treatment for patients with diffuse large B-cell lymphoma (DLBCL).1,2
There has been much discussion about this trial and its practice changing potential, from the primary efficacy endpoint (progression-free survival [PFS]) to the cost of polatuzumab vedotin compared with vincristine and whether that cost is allayed by the survival benefit and/or the potential for curing patients in the first line setting. In the question-and-answer session that followed his abstract presentation, Tilly received many questions regarding the so called “financial toxicity” of polatuzumab vedotin. There has also been discussion regarding polatuzumab’s efficacy in various subgroups though, despite some interesting trends, POLARIX was not powered for subgroup analyses.1,2
Despite these debates, it appears likely that POLARIX will change the standard of care in first line DLBCL, where R-CHOP fails approximately 40% of patients. The full manuscript of the POLARIX trial was published in the New England Journal of Medicine, and the timing coincided with Tilly’s abstract presentation at ASH. Continue reading for a summary of the POLARIX trial.2
Study design
POLARIX was a double-blind, placebo-controlled, international phase III trial in which patients were randomized 1:1 to polatuzumab vedotin (pola)-R-CHP or R-CHOP. Eligible patients were between 18 and 80 years of age with treatment-naïve, CD20-positive DLBCL and had an Eastern Cooperative Oncology Group Performance Status of 0 to 2, an International Prognostic Index (IPI) score between 2 and 5, and adequate organ function. Patients with a history of indolent lymphoma and patients with known central nervous system (CNS) involvement were excluded.2
Randomization was stratified based on IPI score, bulky disease status, and geographic location.
The primary endpoint was investigator-assessed PFS; key secondary endpoints included investigator-assessed event-free survival (EFS) and positron emission tomography and computed tomography-based complete response (CR) at the end of treatment. The primary safety endpoint was a comparison of the incidence of adverse events (AEs) between the two treatment arms.
Treatment
Eight 21-day treatment cycles were planned, with patients receiving either pola-R-CHP or R-CHOP for the first 6 cycles (Figure 1), and all patients receiving rituximab monotherapy during Cycles 7 and 8.
Figure 1. Treatment schema*
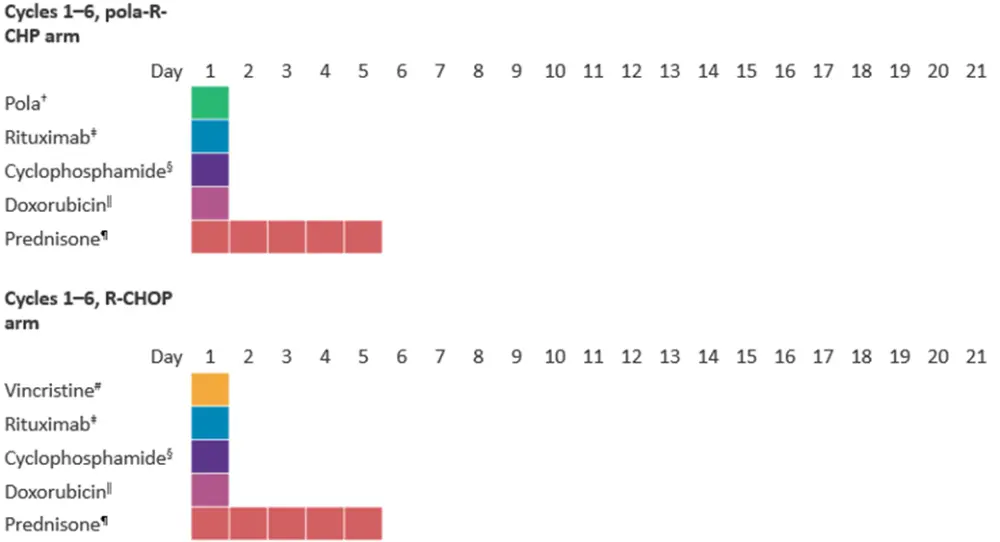
*Data from Tilly, et al.2
†Intravenous polatuzumab vedotin 1.8 mg/kg body weight + placebo matching intravenous vincristine.
‡375 mg/m2 intravenous.
§750 mg/m2 intravenous.
‖50 mg/m2 intravenous.
¶100 mg oral.
#Intravenous vincristine 1.4 mg/m2 (max 2 mg) + placebo matching polatuzumab vedotin.
A few additional notes on treatment:
- CNS prophylaxis with intrathecal chemotherapy was permitted.
- Granulocyte colony-stimulating factor was required during the first 6 cycles.
- Consolidative radiotherapy for bulky/extranodal sites was permitted at the discretion of the investigator.
Baseline characteristics
Of the 1,063 patients screened for eligibility, 879 were randomized, with 440 assigned to the pola-R-CHP arm and 439 assigned to the R-CHOP arm: this was the intention-to-treat (ITT) population. Baseline characteristics were similar between the two groups, and the median age of the overall cohort was 65 years (Table 1).
Most patients received all six doses of polatuzumab vedotin (91.7%) or vincristine (88.5%) in the pola-R-CHP and R-CHOP arms, respectively, with 88.0% and 85.9% receiving all eight treatment cycles. Also of note:
- In the pola-R-CHP group, 11 patients (2.5%) received preplanned radiotherapy after completion of the trial treatment, and 72 patients (16.4%) received CNS prophylaxis.
- In the R-CHOP group, 18 patients (4.1%) received preplanned radiotherapy, and 86 patients (19.6%) received CNS prophylaxis.
Table 1. Baseline patient characteristics in the ITT population*
|
ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; ITT, intention-to-treat; pola-R-CHP, polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. |
||
|
Characteristic, % (unless otherwise specified) |
Pola-R-CHP |
R-CHOP |
|---|---|---|
|
Median age (range), years |
65 (19‒80) |
66 (19‒80) |
|
Age >60 years |
68.2 |
70.2 |
|
Female sex |
45.7 |
46.7 |
|
Geographic region |
||
|
Western Europe, United States, Canada, Australia |
68.6 |
68.6 |
|
Asia |
18.4 |
18.0 |
|
Rest of world |
13.0 |
13.4 |
|
Ann Arbor stage |
||
|
I or II |
10.7 |
11.8 |
|
III or IV |
89.3 |
88.2 |
|
Number of extranodal sites |
||
|
0 or 1 |
51.6 |
51.5 |
|
≥2 |
48.4 |
48.5 |
|
Bulky disease |
43.9 |
43.7 |
|
ECOG Performance Status score |
||
|
0 or 1 |
85.0 |
82.7 |
|
2 |
15.0 |
17.1 |
|
Elevated lactate dehydrogenase level |
66.1 |
64.7 |
|
IPI score |
||
|
2 |
38.0 |
38.0 |
|
3 to 5 |
62.0 |
62.0 |
|
Median time from initial diagnosis to treatment initiation, days |
26 |
27 |
|
Cell of origin |
||
|
Germinal-center B-cell-like subtype |
55.8 |
49.7 |
|
Activated B-cell-like subtype |
30.9 |
35.2 |
|
Unclassified |
13.3 |
15.1 |
|
Double-expressor lymphoma |
38.4 |
41.3 |
|
Double-hit or triple-hit lymphoma |
7.9 |
5.7 |
Results
Primary efficacy end point: PFS
With a median follow-up of 28.2 months (range, 0.1 to 43.4 months), the risk of progression, relapse or death was significantly lower in the pola-R-CHP arm (p = 0.02), and the estimated percentage of patients with PFS at 2 years was 6.5 points higher in the pola-R-CHP group (76.7%) versus the R-CHOP group (70.2%) (Table 2).
Table 2. Efficacy results in the ITT population*
|
ITT, intention-to-treat; pola-R-CHP, polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. |
|||
|
Variable, % (unless otherwise specified) |
Pola-R-CHP |
R-CHOP |
p value |
|---|---|---|---|
|
Progression-free survival |
|||
|
Patients who died or had progression or relapse |
24.3 |
30.5 |
0.02 |
|
Earliest event, n |
|||
|
Death |
19 |
20 |
— |
|
Progression or relapse |
88 |
114 |
— |
|
Estimate at 1 year |
83.9 |
79.8 |
— |
|
Estimate at 2 years |
76.7 |
70.2 |
— |
|
Event-free survival |
|||
|
Patients who died, had progression or relapse, or had other events |
25.5 |
31.4 |
0.02 |
|
Earliest event, n |
|||
|
Death |
18 |
20 |
— |
|
Progression or relapse |
86 |
106 |
— |
|
Other |
8 |
12 |
— |
|
Estimate at 2 years |
75.6 |
69.4 |
— |
|
Response status at treatment completion |
|||
|
Overall response |
85.5 |
83.8 |
— |
|
Complete response |
78.0 |
74.0 |
— |
|
Partial response |
7.5 |
9.8 |
— |
|
Stable disease |
1.8 |
1.4 |
— |
|
Progressive disease |
5.0 |
6.4 |
— |
|
Not evaluated or data missing |
7.7 |
8.4 |
— |
|
Overall survival |
|||
|
Patients who died |
12.0 |
13.0 |
0.75 |
|
Estimate at 2 years |
88.7 |
88.6 |
— |
|
Disease-free survival |
|||
|
Number of patients who could be evaluated |
381 |
363 |
— |
|
Patients who died or had relapse |
16.3 |
21.8 |
— |
|
Earliest event, n |
|||
|
Death |
8 |
13 |
— |
|
Relapse |
54 |
66 |
— |
An exploratory subgroup analysis showed that there was no clear benefit with pola-R-CHP in the following subgroups:
- patients ≤60 years of age
- patients with germinal-center B-cell-like subtype
- patients with bulky disease
- patients with lower IPI scores
Secondary end points
The relative risk of events was lower in the pola-R-CHP group than in the R-CHOP group, with 2-year EFS estimates of 75.6% and 69.4%, respectively (p = 0.02; Table 2). The percentage of patients achieving CR was not significantly different between the two groups (78.0% vs 74.0%, p = 0.16), though patients in the pola-R-CHP group who achieved a CR as best response were more likely to have persistence of remission compared to the same group of patients in the R-CHOP arm. OS was not significantly different between the groups.
Subsequent therapy for lymphoma
Regarding subsequent lymphoma treatment, 22.5% (99/440) of patients in the pola-R-CHP group and 30.3% (133/439) of patients in the R-CHOP group had received at least one subsequent lymphoma treatment at the time of data cutoff. Fewer patients in the pola-R-CHP group versus the R-CHOP group received subsequent:
-
- Planned or unplanned radiotherapy (9.3% vs 13.0%)
- Systemic therapy (17.0% vs 23.5%)
- Stem-cell transplantation (3.9% vs 7.1%)
- Chimeric antigen receptor T-cell therapy (2.0% vs 3.6%)
After disease progression, eight patients in the R-CHOP group received polatuzumab vedotin as part of a subsequent regimen.
Safety
The most common Grade 3–4 AEs in the pola-R-CHP and R-CHOP arms were neutropenia (28.3% and 30.8%), febrile neutropenia (13.8% and 8.0%), and anemia (12.0% and 8.4%) (Table 3). AEs of any grade occurred in 97.9% of patients in the pola-R-CHP arm and in 98.4% of patients in the R-CHOP arm. Grade ≥3 AEs occurred in 60.7% and 59.8%, respectively, with Grade 5 AEs in 3.0% and 2.5% (most due to infections), and serious AEs in 34.0% and 30.6%, respectively.
Table 3. Most common adverse events† (safety population)*
|
Pola-R-CHP, polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. |
||||
|
Adverse event, % |
Pola-R-CHP |
R-CHOP |
||
|---|---|---|---|---|
|
|
Any grade |
Grade 3–4 |
Any grade |
Grade 3–4 |
|
Peripheral neuropathy |
52.9 |
1.6 |
53.9 |
1.1 |
|
Nausea |
41.6 |
1.1 |
36.8 |
0.5 |
|
Neutropenia |
30.8 |
28.3 |
32.6 |
30.8 |
|
Diarrhea |
30.8 |
3.9 |
20.1 |
1.8 |
|
Anemia |
28.7 |
12.0 |
26.0 |
8.4 |
|
Constipation |
28.7 |
1.1 |
29.0 |
0.2 |
|
Fatigue |
25.7 |
0.9 |
26.5 |
2.5 |
|
Alopecia |
24.4 |
0 |
24.0 |
0.2 |
|
Decreased appetite |
16.3 |
1.1 |
14.2 |
0.7 |
|
Pyrexia |
15.6 |
1.4 |
12.6 |
0 |
|
Vomiting |
14.9 |
1.1 |
14.4 |
0.7 |
|
Febrile neutropenia |
14.3 |
13.8 |
8.0 |
8.0 |
|
Headache |
12.9 |
0.2 |
13.0 |
0.9 |
|
Cough |
12.9 |
0 |
12.1 |
0 |
|
Decreased weight |
12.6 |
0.9 |
11.9 |
0.2 |
|
Asthenia |
12.2 |
1.6 |
12.1 |
0.5 |
|
Dysgeusia |
11.3 |
0 |
13.0 |
0 |
Overall, 4.4% of patients in the pola-R-CHP arm discontinued polatuzumab vedotin and 5.0% of patients in the R-CHOP arm discontinued vincristine due to AEs (mostly neurologic events in both groups).
Peripheral neuropathy of any grade and peripheral neuropathy Grade ≥2 was reported at similar rates in both the pola-R-CHP and R-CHOP groups (52.9% vs 52.9% and 13.8% vs 16.7%).
- Median times to onset and resolution in the pola-R-CHP and R-CHOP groups were 2.3 vs 1.9 months and 4.0 vs 4.6 months, respectively.
- 0.2% of patients in the pola-R-CHP group and 0.9% of patients in the R-CHOP group discontinued any treatment due to peripheral neuropathy, and fewer patients in the pola-R-CHP group had a dose reduction due to peripheral neuropathy compared with the R-CHOP group (4.4% vs 8.0%).
Conclusion
Patients in the pola-R-CHP group had a risk of disease progression, relapse or death that was 27% lower compared with the R-CHOP group, and pola-R-CHP was associated with a significant PFS benefit. Again, it is important to note that while this population consisted of patients with intermediate- to high-risk disease, and there was variability in the treatment effect across subgroups, POLARIX was not powered for subgroup analysis, and this will be important to investigate in future trials. EFS was also significantly higher with pola-R-CHP, and remissions appeared to be more durable in the patients who achieved CR in the pola-R-CHP group compared with the R-CHOP group (though the rates of CR did not differ significantly between the two treatment arms). With the median follow-up of 28.2 months, an OS benefit was not observed, though the investigators are hopeful that one will be seen with further follow up.
Your opinion matters
What kind of 1L DLBCL patients should now be treated with Pola-R-CHP (choose all options that apply)?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

