All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
The emerging role of MRD in the treatment of chronic lymphocytic leukemia
Do you know... Which of the following assays have been reported to achieve MRD sensitivity of 10−6 in CLL trials?
Minimal residual disease (MRD) is a highly sensitive biomarker of disease burden and predictor of survival outcomes. More recently, MRD has been implemented as a surrogate endpoint in many hematologic malignancies evaluated in clinical trials, in which presence of undetectable MRD (uMRD) correlates with prolonged progression free survival (PFS) and overall survival (OS).1,2 With the advent of targeted therapies and monoclonal antibody combinations, MRD has also gained an essential role as a surrogate endpoint for the assessment of depth of response and treatment superiority in chronic lymphocytic leukemia (CLL).1,3
The prognostic value of MRD status on survival outcomes has been demonstrated with chemoimmunotherapy and cellular therapies,3 although there is heterogeneity in responses among new targeted therapies.1 In addition, MRD has facilitated faster reporting of treatment outcomes than standard PFS and OS endpoints.1,2 While the prognostic significance of MRD assessment in clinical trials is established, its role in routine clinical practice has not yet been well defined.3
The Lymphoma Hub has previously reported on the use of MRD as a surrogate endpoint in CLL clinical trials. Here, we are pleased to provide an overview of the emerging role of MRD in CLL, including methods of MRD detection, clinical data on MRD significance, MRD-guided treatment strategies, and future perspectives on its utility in the management of CLL.
MRD detection methods1,2,3
There are three methods used for MRD detection at a sensitivity level of <10−4: multicolor flow cytometry (MFC), real-time quantitative polymerase chain reaction (RQ-PCR), and next-generation sequencing.2,3 Although each method has differing sensitivity, target, standardization, advantages, and disadvantages, they can still be used as complementary tools for comprehensive MRD detection (Figure 1).1,3 The final choice of MRD detection method will depend on each laboratory set up and the purpose for MRD monitoring.2
Figure 1. Comparison of MRD assessment detection methods*
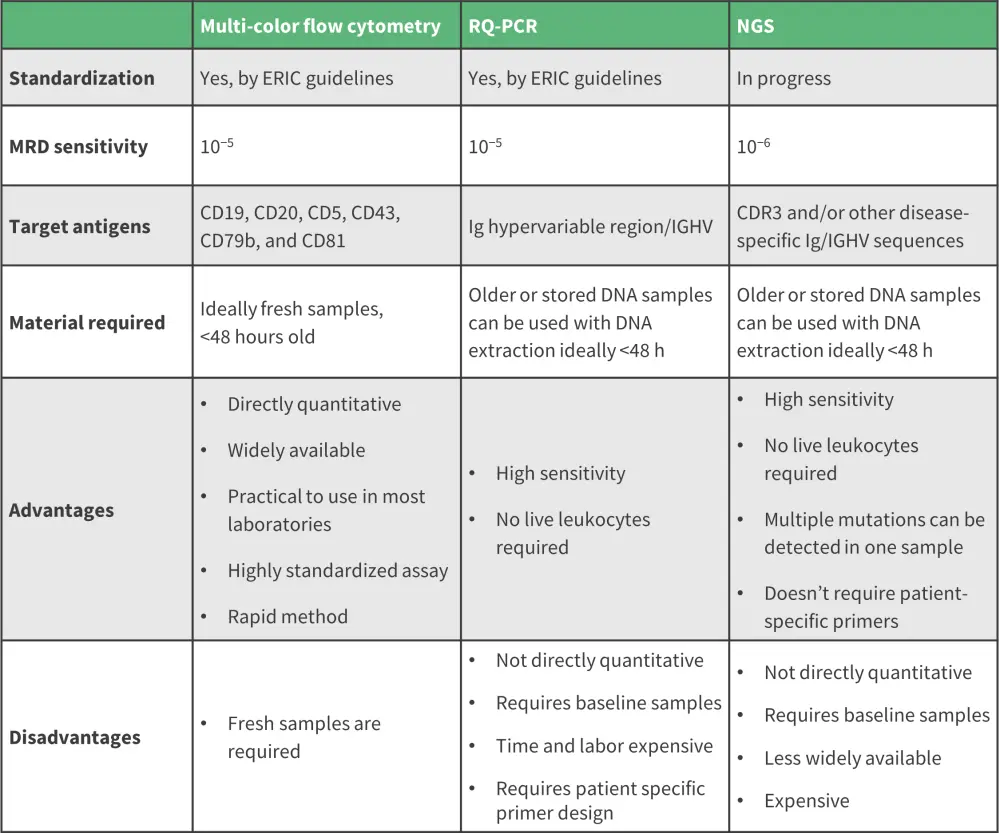
ERIC, European Research Initiative on CLL; IGHV, immunoglobulin hypervariable region; MRD, minimal residual disease; NGS, next-generation sequencing; RQ-PCR, real-time quantitative polymerase chain reaction.
*Adapted from Benintende, et al.1; Fisher, et al.2; and Furstenau, et al.3
Multi-color flow cytometry
MFC is a widely used assay which has been optimized in CLL over several years.3 It employs the use of fluorescently labelled antibodies to facilitate the phenotyping of CLL cells by specific surface antigen expression; it can achieve a limit of detection of 10−5 on a single-tube.1,3 The protocol can be extended beyond the core six-color, one tube method to incorporate additional novel markers such as ROR1, CD200, and CD160 to increase sensitivity and limit of detection.1
Real-time quantitative polymerase chain reaction
Allele-specific oligonucleotide PCR uses patient-specific primers to identify disease-specific immunoglobulin genes within hypervariable regions (IGHV) relative to a CLL clone. For this method, an MRD detection limit between 10−4 and below 10−5 has been reported.1,3
Next-generation sequencing
Although not yet widely validated, next-generation sequencing is an emerging MRD assessment tool which has demonstrated comparable MRD detection results to flow cytometry, down to 10−4 sensitivity.1,2 This method uses consensus primers for amplification of all IGH genes followed by detection of disease-specific IGH gene segments.3 Similar to RQ-PCR, it has the advantage of using DNA samples, including older or stored samples.2,3 It presents the most reliable method for the prediction of survival outcomes and provides higher specificity and sensitivity, with a reported MRD limit of detection of 10−6 being achieved.1,2
Peripheral blood vs bone marrow for MRD detection1
The presence of leukemic cells can be detected in peripheral blood (PB) and bone marrow (BM). In patients with CLL, MRD has shown prognostic significance for PFS and OS in both PB and BM samples following treatment. However, MRD levels and prognostic ability could differ between sampling sites; therefore, optimal choice depends on various factors, including the timing of sampling and treatment status.
PB and BM MRD assessments are roughly similar in 85% of cases at a sensitivity level of 10−4. However, for rituximab, the concordance decreases to 79%, with a higher MRD sensitivity and prognostic effect in BM versus PB. Generally, PB rather than BM sampling is more common in routine clinical practice, owing to the invasive procedure associated with collecting BM samples.1
MRD assessment in clinical trials1,2,3
MRD assessment after chemoimmunotherapy1,3
At the recommended sensitivity of 10−4, MRD can independently predict survival outcomes in patients receiving first-line chemoimmunotherapy, with several studies demonstrating its prognostic significance with various combination regimens.
- CLL8 trial (NCT00281918): MRD-evaluable patients receiving fludarabine, cyclophosphamide, and rituximab (FCR) achieved a higher uMRD (<10−4) PB level compared with those treated with FC alone (63% vs 35%); low MRD levels were predictive of both PFS and OS outcomes.1,3
- CLL10 trial (NCT00769522): a higher uMRD was observed in patients treated with FCR vs bendamustine rituximab in both the MRD-evaluable cohort (74 vs 63%) and the intention-to-treat cohort (49% vs 38%).3
- CLL11 trial (NCT01010061): the prognostic significance of PB MRD was demonstrated in patients treated with chlorambucil and obinutuzumab versus chlorambucil and rituximab; those achieving uMRD had prolonged PFS and event-free survival compared with MRD-positive group.3
MRD assessment after Bruton kinase inhibitors1,3
The Bruton’s tyrosine kinase inhibitor (BTKi) ibrutinib was initially indicated as second-line therapy in relapsed CLL and, more recently, as first-line therapy in newly diagnosed CLL.1 Despite its overall clinical efficacy and excellent survival outcomes, only a minority of patients treated with ibrutinib monotherapy achieved MRD negativity.3 A study reported PB MRD negativity rates of only 10.2% in both treatment naïve and relapsed/refractory patients after 5 years of continuous ibrutinib monotherapy1,3; CR rates were 37.5% and 21.3%, respectively, in the low MRD (<10−2) vs high MRD (≥10−2) group.1,3
On the other hand, in the HELIOS trial (NCT01611090) evaluating ibrutinib with bendamustine rituximab vs bendamustine rituximab alone, ibrutinib + bendamustine rituximab yielded uMRD rates.3 The clinical data on MRD from selected BTKi studies are shown in Figure 2.1,3
MRD data have not been reported for other BTKis such as zanubrutinib and acalabrutinib. However, the ongoing CLL2-BCG trial (NCT02445131), investigating idelalisib with obinutuzumab, and the CLLRUmbrella1 and 2 trials (NCT02968563; NCT02983617), assessing dual kinase inhibition (PI3K/BTK) in combination with obinutuzumab, will provide further efficacy data.3
Figure 2. Key MRD data from selected BTKi trials*
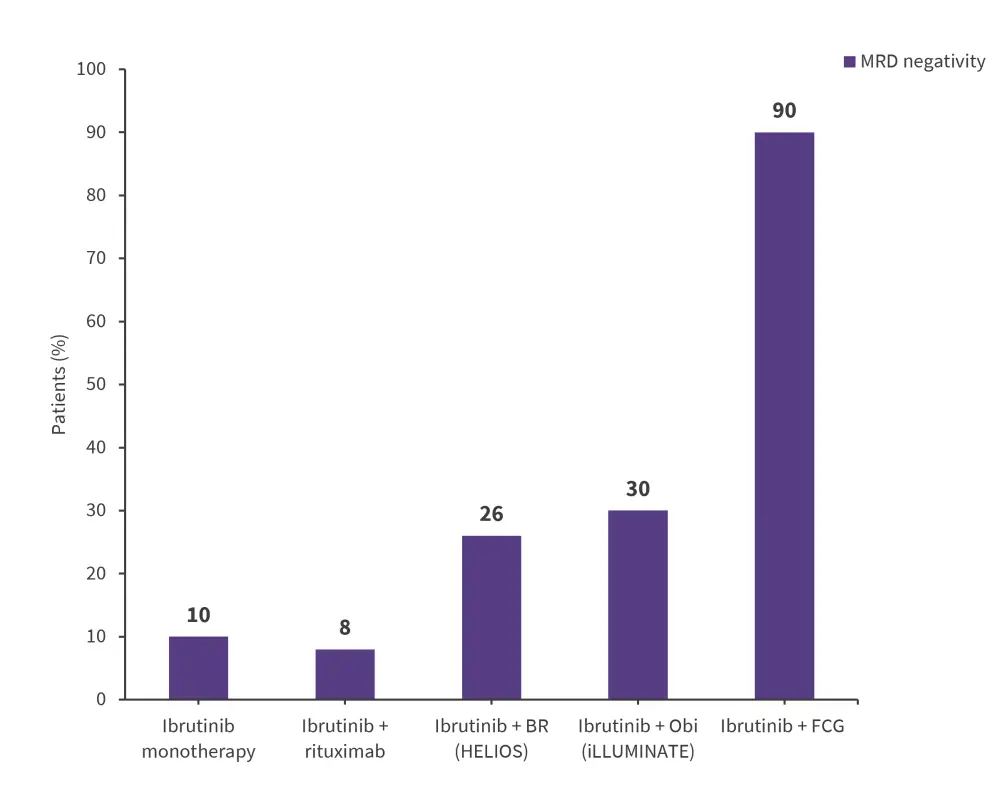
BR, bendamustine rituximab; BTKi, Bruton’s tyrosine kinase inhibitor; FCG, fludarabine, cyclophosphamide; MRD, minimal residual disease, Obi, obinutuzumab.
*Adapted from Benintende, et al.1 and Furstenau, et al.3
MRD assessment after BCL2 inhibitors1,3
Venetoclax, a BCL2 inhibitor, has demonstrated high MRD negativity rates as monotherapy and even higher rates when combined with other agents.1,3 The M13-982 (NCT01889186) and M14-032 (NCT02141282) trials reported a PB MRD negativity rate of 20% in patients with relapsed/refractory CLL + 17p deletion and 42% in patients with CLL who did not respond to a BTKi. The pooled MRD analysis showed a higher 24-month PFS rate for uMRD vs intermediate MRD vs high MRD (92.8% vs 84.3% vs 63.2%).3
High MRD negativity rates have been achieved by several trials investigating venetoclax combined regimens, as shown in Figure 3. In the MURANO (NCT02005471) and CLL14 (NCT02242942) trials, high MRD negativity in the treatment vs comparator arm also translated into a prolonged PFS.1,3
Figure 3. High MRD negativity rates in venetoclax-based regimens*
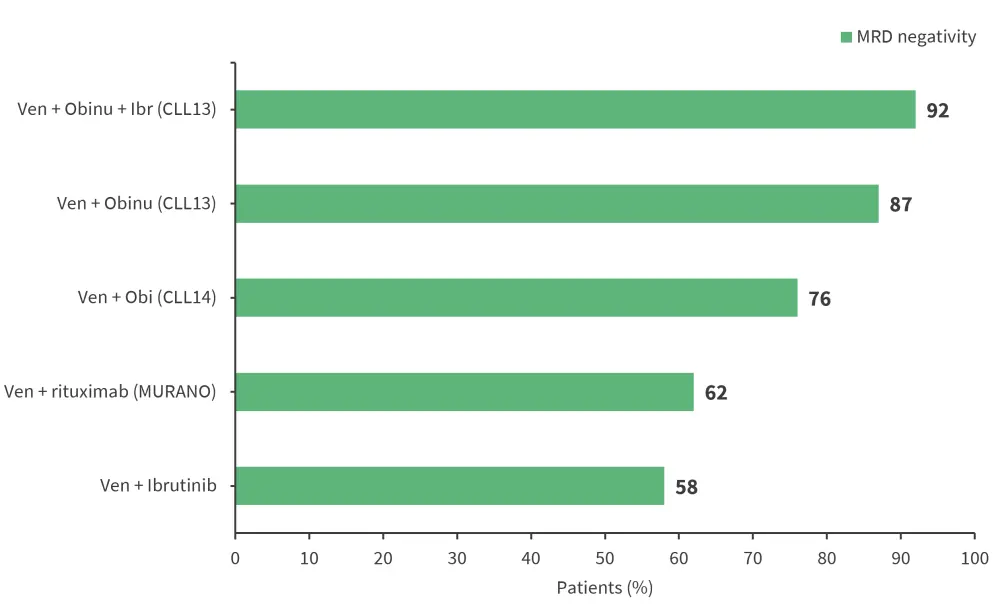
BR, bendamustine rituximab; FCR, fludarabine, cyclophosphamide, and rituximab; MRD, minimal residual disease, obi, obinutuzumab.
*Adapted from Benintende, et al1 and Furstenau, et al3
Using MRD to guide treatment decisions: future perspectives1,3
Beyond its utility as a surrogate endpoint, MRD can be further employed in clinical trial design to facilitate individualized treatment strategies. Interim MRD analysis could identify patients eligible for treatment de-escalation and/or cessation to mitigate treatment-related toxicities, alongside genetic assessments to identify patients who may benefit from further treatments (Figure 4).3
Figure 4. Role of MRD in clinical practice*
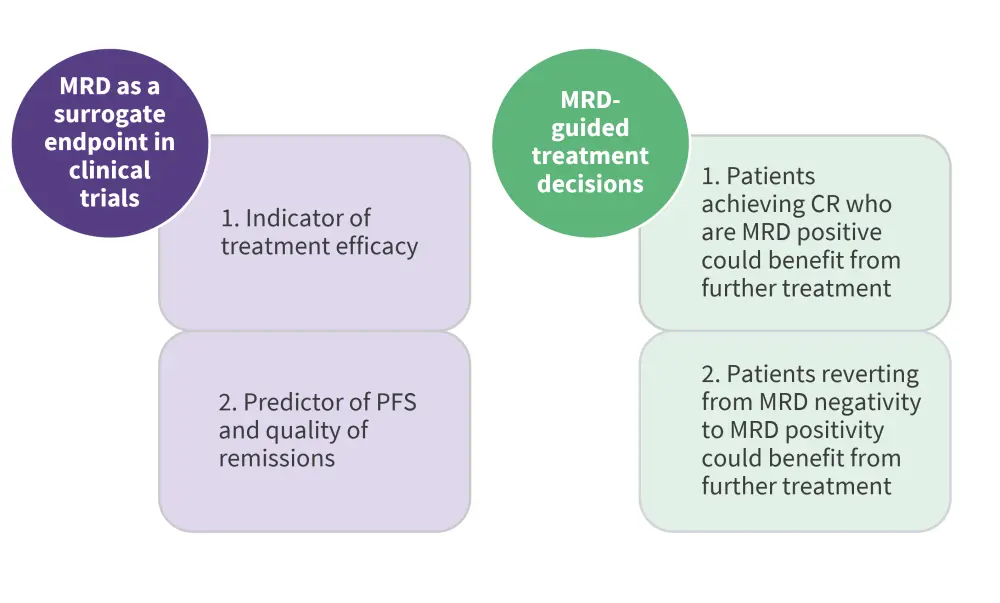
CR, clinical remission; MRD, minimal residual disease; PFS, progression-free survival
*Adapted from Benintende, et al.1
Examples of MRD-guided treatment strategies include a trial (NCT02629809) evaluating first-line treatment with ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) in patients with a favourable genetic profile.3 BM MRD was assessed following three cycles of iFCG; those with uMRD received three additional cycles of ibrutinib and obinutuzumab and six more cycles of ibrutinib, while those not achieving uMRD received nine additional cycles of ibrutinib and obinutuzumab. In those who reached uMRD at 1-year, therapy was stopped.3 Another example is the CAPTIVATE trial (NCT02910583) which evaluated ibrutinib plus venetoclax in an MRD-guided cohort (Figure 5).1
Figure 5. MRD-guided strategy in CAPTIVATE study*
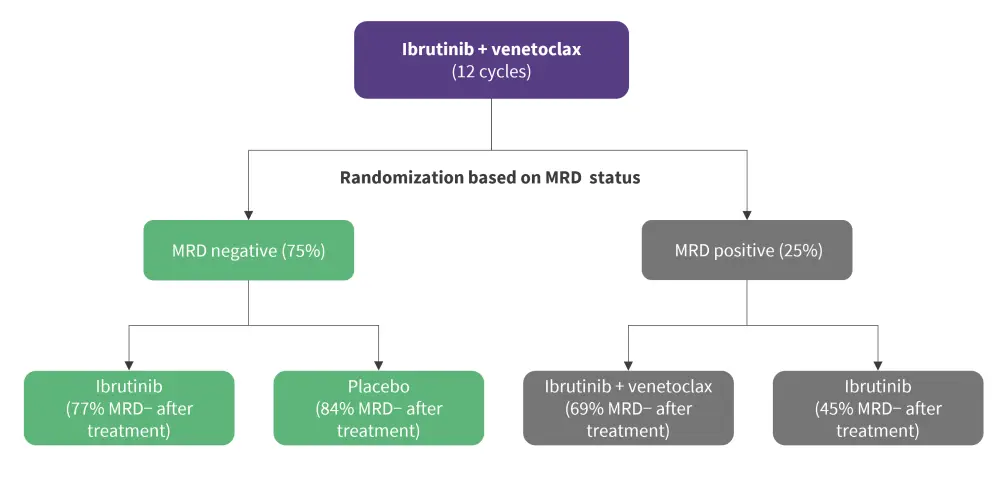
MRD, minimal residual disease; MRD-, MRD negativity
*Adapted from Benintende, et al1
Conclusion
This review highlights the significant role of MRD as a surrogate endpoint in CLL trials and its increasing utility to facilitate personalized treatment strategies. While its use was discouraged with the introduction of ibrutinib, venetoclax monotherapy and combination regimens have helped to demonstrate the key role of MRD as a predictor of event-free survival. Although MRD is not presently included in routine clinical practice, it may be implemented in future guidelines to define patient response and prognostic outcomes. Further studies are warranted, both to refine and develop more sensitive methods of MRD detection and to elucidate its role in different treatment modalities and genetic subgroups.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

