All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
The prognostic value of PET scan in the treatment of DLBCL
Positron emission tomography (PET)-computed tomography (CT) is considered as having a prognostic value in the treatment of diffuse large-B cell lymphoma (DLBCL). A positive or negative PET-CT scan after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and disease stage-dependent consolidative radiation therapy (RT), may guide treatment decisions, improve outcomes, and safeguard patients from RT-associated adverse events.1 The controversy around the use of RT in patients with advanced-stage DLBCL remains, and thus, there is a great need for distinguishing patients requiring RT from those with low-risk disease that could be spared.2
Two different studies have investigated the outcomes of PET-guided treatment decisions in patients with limited-stage DLBCL (S1001 study, NCT01359592)1, and those with advanced-stage DLBCL2. We hereby combine and summarize the key points of those two studies.
Limited-stage DLBCL1
DLBCL appears as a limited-stage disease in 25–30% of patients and is associated with a better overall survival (OS) compared with advanced-stage disease. Three cycles of R-CHOP followed by RT represent the standard of care based on the recommendations of the National Comprehensive Cancer Network (NCCN). Persky et al., investigated in a prospective phase II trial (S1001) the impact of subsequent therapies after a positive or negative PET in patients ≥ 18 years of age who
- were previously untreated
- had non-bulky (< 10 cm) stage I/II CD20-positive DLBCL
- had a World Health Organization performance status (WHO PS) of 0–2
- had a negative bone marrow biopsy within 6 weeks of registration
The primary endpoint was progression-free survival (PFS) at 5 years, and secondary endpoints included OS rate, PET subgroup outcome analysis, safety, and response rates.
Methods
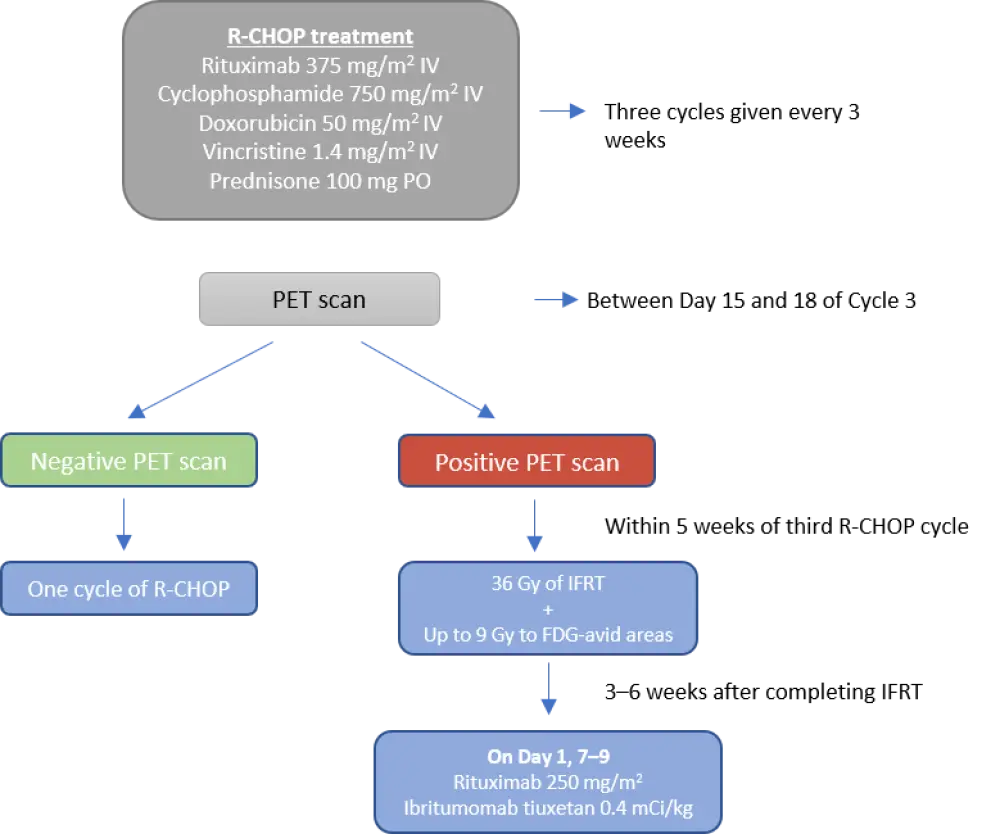
Patients were treated with three cycles of standard R-CHOP treatment based on the dosing schedule shown in Figure 1, and then had an interim PET-CT scan at Day 15 and 18 of Cycle 3. A final PET scan was done 12 weeks after the end of treatment, and patients were monitored every 6 months during the first 2 years, and annually thereafter for up to 7 years or death.
Figure 1. Treatment schedule1
Patients
The number of eligible patients included in the trial was 132, with a median age of 62 years (range, 18–86). Patient characteristics are summarized in Table 1.
Table 1. Patient characteristics in the S1001 trial1
|
ABC, activated B-cell; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell; HGBL, high-grade B-cell lymphoma; LDH, lactate dehydrogenase; NOS, not-otherwise specified, WHO PS, World Health Organization performance status. |
|
|
Characteristic |
N = 132 |
|---|---|
|
Age, years Median (range) > 60 years, n (%) |
62 (18–86) 71 (54) |
|
WHO PS, n (%) 0 1 2 |
89 (67) 39 (30) 4 (3) |
|
Disease stage, n (%) I II |
82 (62) 50 (38) |
|
Elevated LDH, n (%) |
19 (14) |
|
Head and neck-only involvement, n (%) |
87 (66) |
|
Extranodal involvement, n (%) |
57 (43) |
|
Subtype, n (%) DLBCL, NOS HGBL with MYC and BCL2 and/or BCL6 rearrangements HGBL, NOS T cell/histiocyte-rich large B-cell lymphoma Pathology not performed |
95 (72) 4 (3) 22 (17) 2 (2) 9 (7) |
|
Cell of origin, n (%), n = 87 GCB ABC Unclassifiable |
59 (68) 20 (23) 8 (9) |
Of the 132 patients, 128 had their interim PET scans reviewed:
- 110 were negative
- 18 patients were positive; four due to infection but treated as PET-negative with one more R-CHOP cycle
- Of the 14 PET-positive patients (11%), 12 received involved-field radiation therapy (IFRT) and ibritumomab tiuxetan with eight of them achieving complete response (67%; CR), and four achieving partial response (33%; PR)
Outcomes
Median time from diagnosis to treatment was 31 days. Almost all patients (98%) completed the R-CHOP treatment as planned. Median follow-up was 4.92 years (range, 1.1–7.7). Survival outcomes and response rates are shown in Table 2.
Table 2. Outcomes from the S1001 trial1
|
CR, complete response; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PR, partial response; SD, stable disease. |
|
|
Outcome, % |
N = 128 |
|---|---|
|
CR |
92 |
|
PR |
4 |
|
SD |
1 |
|
5-year PFS, % (95% CI) PET-positive PET-negative |
87 (79–92) 86 (54–96) 89 (80–94) |
|
5-year OS, % (95% CI) PET-positive PET-negative |
89 (82–94) 85 (52–96) 91 (84–95) |
Six patients had disease progression with a median time-to-progression (TTP) of 1.1 years (range, 0.2–6.2), and three died in the PET-positive subgroup. Eleven patients with a median age of 80 years (range, 56–86) died due to causes not related to lymphoma. The most common Grade ≥ 3 adverse events were of hematological nature (42%). All Grade ≥ 3 adverse events are presented in Table 3. Two Grade 3–4 neutropenia, and three Grade 3–4 thrombocytopenia events were seen in patients receiving IFRT and ibritumomab tiuxetan.
Table 3. Grade ≥ 3 adverse events from the S1001 trial1
|
AE, adverse event; UTI, urinary tract infection; WBC, white blood cell |
|
|
AE |
n (%) |
|---|---|
|
Neutrophil count reduced |
41 (31) |
|
WBC reduced |
36 (27) |
|
Lymphocyte count reduced |
23 (17) |
|
Febrile neutropenia |
14 (10) |
|
Anemia |
10 (8) |
|
Platelet count reduced |
10 (8) |
|
Fatigue |
3 (2) |
|
UTI |
3 (2) |
|
Lung infection |
2 (2) |
|
Peripheral neuropathy |
2 (2) |
|
Nausea |
1 (1) |
Conclusion
In this trial, PET-adapted therapy led to favorable outcomes for both PET-negative and PET-positive patients in the long-term. PET-negative patients who were spared from RT and received an additional cycle of R-CHOP had similar PFS and OS results to those with a positive PET scan who received consolidative RT. These findings suggest that PET can guide treatment decisions in patients with limited-stage DLBCL.
Advanced-stage DLBCL2
A common approach for the treatment of advanced-stage DLBCL includes six to eight cycles of R-CHOP with consolidative RT considered in patients with bulky disease or residual disease following immunochemotherapy. Freeman et al.2 conducted a retrospective study to evaluate the value of end-of-treatment (EOT) PET scanning for considering RT consolidation in advanced-stage DLBCL patients based on a 14-year experience.
Methods and patients
Patients included in this study were ≥ 18 years of age with advance-stage DLBCL and must have received R-CHOP followed by EOT PET within 4–6 weeks of therapy completion. Three-year estimates for TTP, and OS outcomes were compared among different subgroups.
The number of patients included in the analysis was 723 with a median age of 65 years (range, 18–89) and the majority (74%) had Ann Arbor disease stage III–IV. Upon completion of therapy, 72% of patients were PET-negative while 28% had a positive PET scan. The vast majority of patients (94%) received 6 cycles of R-CHOP. Patient characteristics for the total population and by PET subgroups are provided in Table 4.
Table 4. Patient characteristics among groups2
|
ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PET, positron emission tomography. |
||||
|
Characteristic, |
Study population |
PET-negative |
PET-positive |
p value |
|---|---|---|---|---|
|
Age > 60 years |
460 (64) |
333 (64) |
127 (62) |
0.49 |
|
ECOG PS 2–4 |
299 (41) |
203 (41) |
96 (47) |
0.15 |
|
Stage III–IV |
534 (74) |
389 (75) |
145 (70) |
0.18 |
|
B-symptoms |
320 (44) |
207 (40) |
113 (55) |
< 0.001 |
|
Elevated LDH |
393 (54) |
257 (54) |
136 (69) |
< 0.001 |
|
Bulky site ≥ 10 cm |
285 (39) |
172 (33) |
113 (55) |
< 0.001 |
|
Skeletal involvement |
142 (20) |
103 (20) |
39 (19) |
0.76 |
|
Craniofacial involvement |
41 (6) |
33 (6) |
8 (4) |
0.19 |
|
Marrow involvement |
77 (11) |
63 (12) |
14 (7) |
0.03 |
|
Extranodal sites > 1 |
221 (31) |
165 (44) |
56 (38) |
0.15 |
|
Hemoglobin < 110 g/L |
191 (26) |
124 (26) |
67 (35) |
0.02 |
|
IPI 3–5 |
377 (52) |
260 (55) |
117 (59) |
0.33 |
Outcomes
With a median follow-up of 4.3 years (range, 0.9–14.2), the following outcomes were observed for PET-negative versus PET-positive patients:
- 3-year TTP: 83% (95% CI, 80–86) vs 56% (95% CI, 49–63), p < 0.001
- 3-year OS: 87% (95% CI, 84–90) vs 64% (95%, 57–70), p < 0.001
Of the 206 patients with an EOT PET-positive scan, 53% received consolidative RT. The following outcomes were observed for this subgroup:
- 3-year TTP was 76% (95% CI, 66–83) in patients who received consolidative RT and 34% (95% CI, 25–44) in patients who did not receive RT (p < 0.01). There was no statistically significant difference between PET-positive patients treated with RT and PET-negative patients in terms of TTP (p = 0.3)
- 3-year OS were:
- 87% (95% CI, 84–90) for patients with negative PET scan
- 80% (95% CI, 71–87) for patients with positive PET scan treated with RT
- 44% (95% CI, 34–54) for patients with positive PET scan not treated with RT
Of the patients who did not receive RT in the PET-positive group, 30% did not relapse compared with 27.5% of patients in the RT receiving PET-positive group.
Conclusions
This study showed that a negative EOT PET scan without RT consolidation was associated with a 3-year relapse-free survival in 83% of patients with advanced-stage DLBCL. This indicates that such patients with a negative EOT PET can be spared from RT and its side effects without compromising their outcomes.
The results from both studies presented above, are supportive of an imaging-guided treatment approach where RT consolidation is decided based on PET scan results following completion of R-CHOP induction therapy in both limited- and advanced-stage DLBCL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

