All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Updated efficacy data from the phase I dose-escalation trial of glofitamab in patients with R/R NHL
Featured:
Patients with relapsed/refractory non-Hodgkin lymphoma (R/R NHL) have limited treatment options, often resulting in poor outcomes. Bispecific antibodies that bind to both CD3 on T cells, and CD20 on malignant B cells have shown high response and complete remission rates in patients with R/R NHL. Glofitamab is an anti-CD20 and anti-CD3 bispecific antibody currently being investigated in a phase I dose-escalation trial (NCT03075696) for use in patients with R/R NHL. The Lymphoma Hub has previously reported preliminary results from this trial, and more recently interviewed one of the investigators, Carmelo Carlo-Stella, Humanitas University, Milan, IT, on how step-up dosing fared when compared with fixed dosing for glofitamab.
During the 26th Congress of the European Hematology Association (EHA2021), an e-poster on updated findings from the phase I dose-escalation trial (NCT03075696) of glofitamab was presented by Carlo-Stella et al.1 Findings as part of the larger cohort from the same trial that included fixed and step-up dosing (SUD), were also published recently in the Journal of Clinical Oncology.2 The fixed dose cohort demonstrated durable complete response and manageable safety profile in patients with R/R NHL.2 The updated results from the SUD cohort are summarized below.
Study design
This was a phase I dose-escalation trial, with SUD of glofitamab in patients with heavily pre-treated R/R NHL. Eligible patients were ≥18 years, had ≥1 prior lymphoma treatment and ≥1 measurable target lesion (>1/5 cm), and had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1.
A total of 52 patients received glofitamab SUD: 17 patients received 2.5/10/16 mg; and 35 patients received 2.5/10/30 mg glofitamab SUD. The SUD schedule was as shown in Figure 1. After Cycle 1 SUD, glofitamab was given every 3 weeks for a fixed duration of treatment up to 12 cycles.
Figure 1. Step-up dosing schedule*
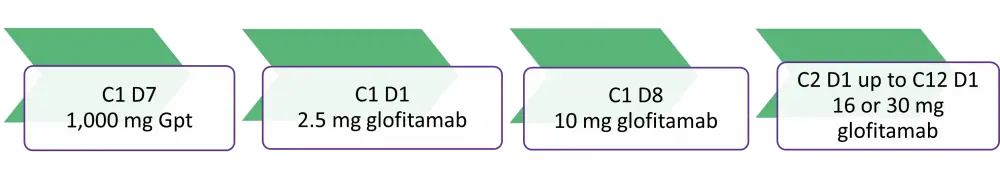
C, cycle; D, day; Gpt, glofitamab fixed dosing with obinutuzumab pre-treatment.
*Adapted from Carlo-Stella et al1 and Hutchings et al.2
- The primary endpoints were safety, tolerability, pharmacokinetics, and the antitumor efficacy (per Lugano criteria) of glofitamab.
- The secondary endpoints included the maximum tolerated, optimal biological, and recommended phase II dose (RP2D) of glofitamab.
Baseline characteristics
The median age of patients in the SUD cohort was 68 years (44−85) and patients had received a median of three prior lines of therapy (range, 1−12). Fifty-four percent of patients had aggressive non-Hodgkin lymphoma (aNHL), and 46% had indolent non-Hodgkin lymphoma (iNHL) (see Table 1).
Table 1. Baseline characteristics*
|
CAR-T, chimeric antigen receptor T cell; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphoma; PI3Ki, phosphoinositide 3-kinase inhibitor. |
|
|
Characteristic |
2.5/10/16 mg and 2.5/10/30 mg cohorts (n = 52) |
|---|---|
|
Prior therapy, % |
|
|
Chemotherapy and anti-CD20 monoclonal antibody |
100 |
|
Autologous stem-cell transplant |
21 |
|
PI3Ki |
10 |
|
CAR-T |
6 |
|
Cancer immunotherapy |
2 |
|
Refractory status, % |
|
|
Refractory to any prior therapy |
85 |
|
Refractory to most recent therapy line |
77 |
|
Refractory to any prior anti-CD20 |
73 |
|
Aggressive NHL, % |
54 |
|
DLBCL |
19 |
|
Transformed FL |
12 |
|
Ritcher transformation |
10 |
|
MCL |
10 |
|
High-grade B-cell lymphoma |
2 |
|
FL Grade 3b |
2 |
|
Indolent NHL, % |
46 |
|
FL Grade 1−3a |
46 |
Results
Data presented from cutoff date of December 01, 2020.
Efficacy1,2
- The best overall response rate (ORR) and complete metabolic response (CMR) rates were 64% and 57%, respectively, in patients with aNHL (see Table 2).
- Patients in the aNHL arm showed a trend of improved response at the RP2D (2.5/10/30mg; n = 14), with a CMR rate of 71%.
- Four of the five patients (80%) with mantle cell lymphoma (2.5/10/16mg, n=2; 2.5/10/30mg, n=2) also attained CMR.
- At a median follow-up of 8 months, patients with aNHL demonstrated ongoing CMR in 13 of 16 patients, with eight CMRs lasting >3 months and five for >6 months.
- Similarly, at a median follow-up of 6 months, patients with iNHL also showed ongoing CMR in 16 of 17 patients, with ten CMRs lasting >3 months and three for >6 months.
Table 2. Response rates*
|
CMR, complete metabolic response; PMR, partial metabolic response; ORR, overall response rate. |
|||
|
Response, % |
All patients |
2.5/10/16 mg cohort |
2.5/10/30 mg cohort |
|---|---|---|---|
|
Aggressive NHL |
n = 28 |
n = 14 |
n = 14 |
|
CMR |
57 |
43 |
71 |
|
PMR |
7 |
7 |
7 |
|
ORR (CMR + PMR) |
64 |
50 |
78 |
|
Indolent NHL |
n = 24 |
n = 3 |
n = 21 |
|
CMR |
71 |
67 |
71 |
|
PMR |
8 |
33 |
5 |
|
ORR (CMR + PMR) |
79 |
100 |
76 |
Safety1,2
- The most common adverse events (AEs) were cytokine release syndrome (CRS) (67%), neutropenia (42%), and pyrexia (39%).
- CRS was frequently Grade 1 or 2 and confined to Cycle 1 and 2 (Figure 2).
- Treatment-related discontinuation was reported in two patients, one due to Grade 4 neutropenia and the other due to Grade 4 colitis (Table 3).
Figure 1. CRS by dose and cycle*
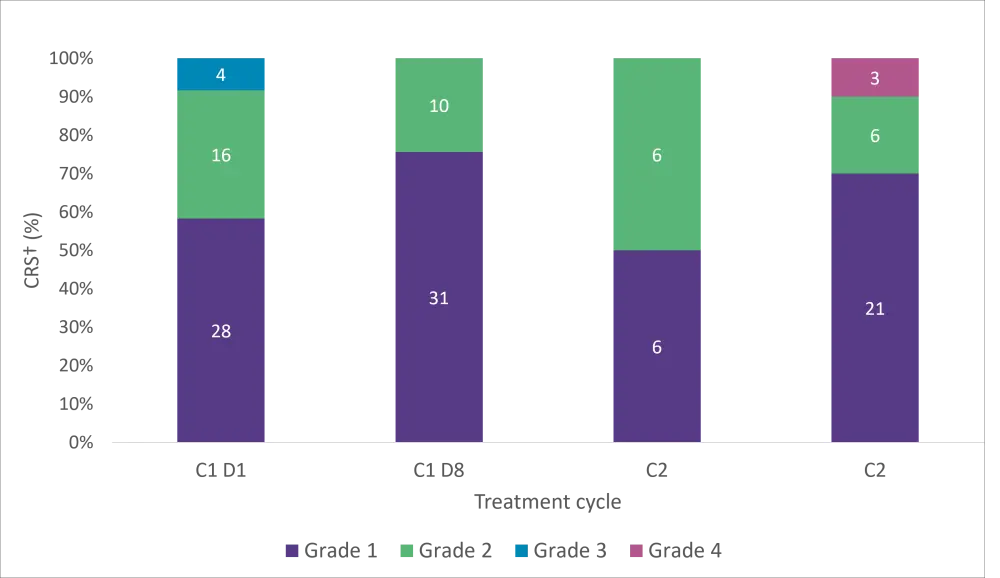
CRS, cytokine release syndrome; C, cycle; D, day.
*Adapted from Carlo-Stella et al.1
†Multiple occurrences of CRS are counted to the highest grade.
Table 3. Adverse events*
|
AE, adverse event. |
|
|
AE, % |
All patients |
|---|---|
|
Any AEs |
98 |
|
Treatment-related |
90 |
|
Serious AEs |
62 |
|
Treatment-related |
56 |
|
Grade 3−4 AEs |
60 |
|
Treatment-related |
40 |
|
Grade 5 AEs |
0 |
|
AEs leading to treatment discontinuation |
4 |
|
Treatment-related† |
4 |
Pharmacodynamics
- Baseline tumor biopsies revealed that clinical responses with SUD of glofitamab were attained across a range of CD20 expression levels in 32 patients and irrespective of the amount of tumor T-cell infiltration in 24 patients.
- There were no significant differences between the response categories.
Conclusion
The updated analysis showed that SUD of glofitamab had early, high, and durable response rates in patients with aNHL and iNHL who were refractory to multiple lines of therapy. Durability of responses was enhanced after completion of the fixed treatment period. The safety profile of SUD of glofitamab was manageable with mostly low-grade AEs confined to either Cycle 1 or 2.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Sheetal Bhurke
Sheetal Bhurke