All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Zanubrutinib versus ibrutinib in R/R CLL/SLL: final ALPINE results
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) is characterized by repeated relapses which result in poor response to therapy and eventually affect survival. The advent of Bruton’s tyrosine kinase (BTK) inhibitors has transformed the treatment of patients with relapsed/refractory (R/R) CLL/SLL, prolonging progression-free survival (PFS) and overall survival (OS) in patients who otherwise have limited treatment options.
Ibrutinib, a first-in-class irreversible BTK inhibitor, is efficacious; however, it is associated with off-target effects due to non-specific kinase inhibition and interference with anti-CD20 monoclonal antibodies. Zanubrutinib, a second-generation BTK inhibitor which is highly selective, was designed to minimize off-target inhibition.1,2
ALPINE (NCT03734016) is a global, phase III, randomized trial of zanubrutinib vs ibrutinib in patients with previously treated CLL/SLL. Interim results of the trial, previously reported by the Lymphoma Hub, found that zanubrutinib met the primary endpoint of non-inferior objective response rate (ORR). The final analysis of the ALPINE trial was presented by Brown at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition in December 20222 and has concurrently been published in New England Journal of Medicine.3 Below, we summarize the key findings.
Study design
Patients with R/R CLL/SLL with ≥1 prior treatment and measurable lymphadenopathy were eligible. Patients were randomized 1:1 to receive either zanubrutinib 160 mg twice daily or ibrutinib 420 mg three times a day. Patients were stratified by age, geographic region, refractory status, and del(17p)/TP53 mutation (mut) status. The design of the final analysis is summarized in Figure 1. ORR non-inferiority and superiority were demonstrated in the ORR interim and final analyses; PFS was tested for non-inferiority under hierarchical testing when 205 events had occurred.
Figure 1. Design of the final analysis*
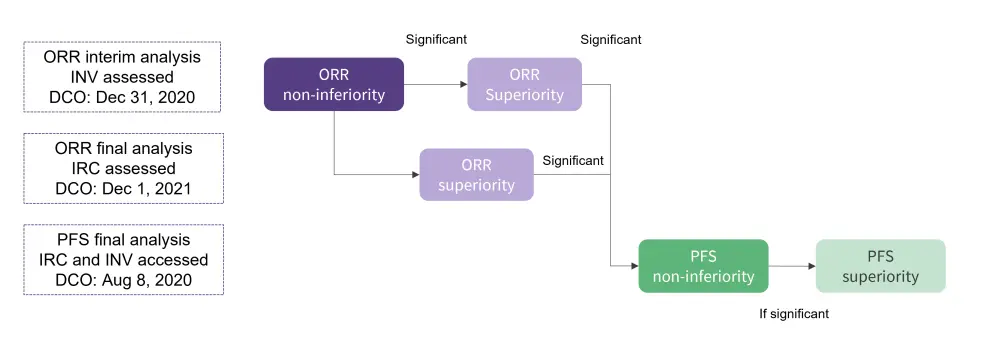
DCO, data cut-off; IRC, independent review committee; INV, investigator; ORR, objective response rate; PFS, prolonging progression-free survival.
*Adapted from Brown, et al.2
The primary endpoint was ORR non-inferiority and superiority (by investigator assessment). The key secondary efficacy endpoint was PFS, with other secondary endpoints including OS, ORR including PR with lymphocytosis (PR-L) or better, and safety parameters including atrial fibrillation/flutter.
Results
Baseline characteristics
Overall, 652 patients from 15 countries were randomized to receive zanubrutinib (n = 327) or ibrutinib (n = 325). The data cut-off was August 8, 2022. Baseline demographics and disease characteristics were well-balanced across both the zanubrutinib and ibrutinib treatment arms (Table 1).
Table 1. Baseline demographics and disease characteristics*
|
ECOG, Eastern Cooperative Oncology Group; IGHV, immunoglobulin heavy chain variable; mut, mutated. |
||
|
Characteristic, % (unless otherwise stated) |
Zanubrutinib arm |
Ibrutinib arm |
|---|---|---|
|
Median (range) age, years |
67 (35–90) |
68 (35–89) |
|
≥65 years |
61.5 |
61.5 |
|
Male |
65.1 |
71.4 |
|
ECOG Performance Status ≥1 |
60.6 |
62.5 |
|
Median prior lines of therapy (range), n |
1 |
1 |
|
>3 prior lines |
7.3 |
9.2 |
|
del(17p) and/or TP53mut |
22.9 |
23.1 |
|
>del(17p) |
13.8 |
15.4 |
|
TP53mut without del(17p) |
9.2 |
7.7 |
|
del(11q) |
27.8 |
27.1 |
|
IGHV mutational status |
||
|
Mutated |
24.2 |
21.5 |
|
Unmutated |
73.1 |
73.5 |
|
Complex karyotype† |
17.1 |
21.5 |
|
Bulky disease (≥5 cm) |
44.3 |
45.8 |
Efficacy
With a median follow-up of 29.6 months, PFS was significantly superior in the zanubrutinib arm vs ibrutinib arm assessed by independent review committee (IRC; p = 0.0024; Figure 2). PFS favored zanubrutinib across all subgroups including prior lines of therapy, del(17p)/TP53mut status, IGHV mutational status, and complex karyotype regardless of IRC or investigator assessment.
Figure 2. Two-year landmark PFS in the zanubrutinib vs ibrutinib arms*
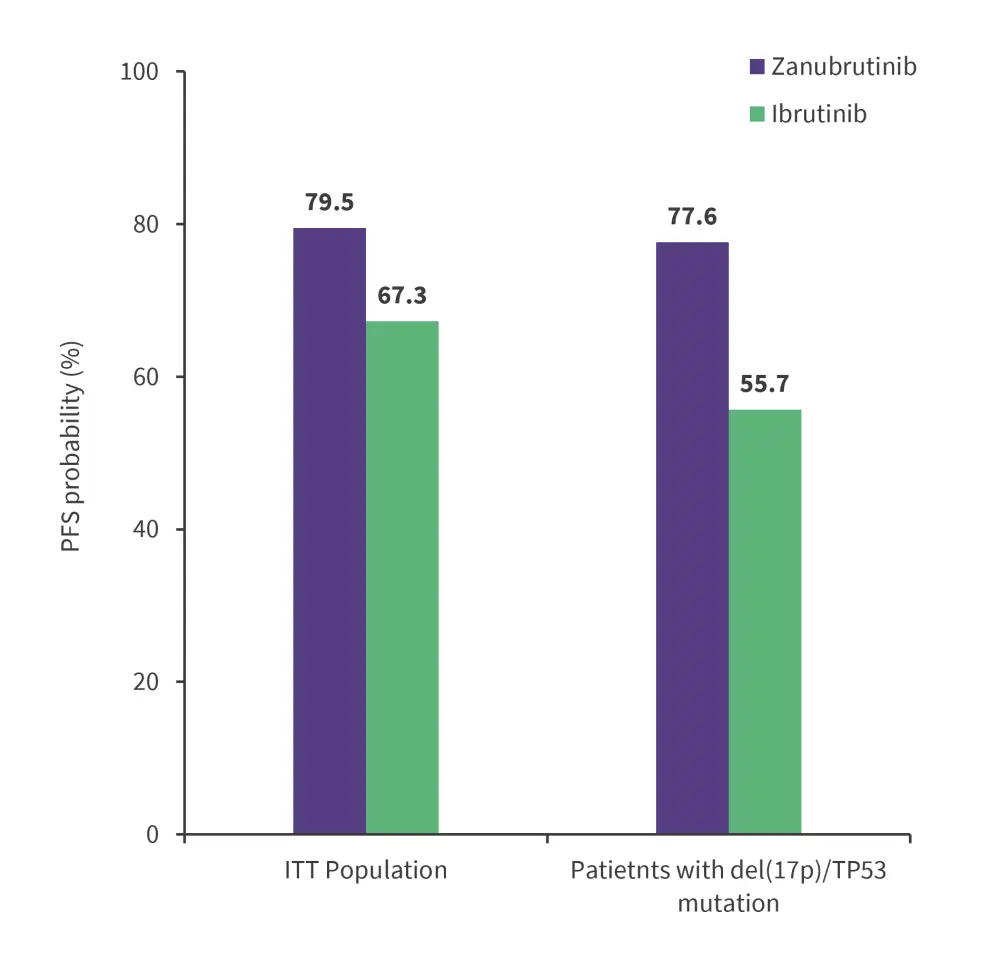
ITT, intent-to-treat; PFS, progression-free survival.
*Adapted from Brown, et al.2
Higher ORR was observed in the zanubrutinib arm vs ibrutinib arm (86.2% vs 75.7%; p = 0.007) when assessed by IRC (Figure 3), with a rate of PR-L or better of 91.7% vs 83.1% (p = 0.001). A lower number of deaths were reported in the zanubrutinib arm vs ibrutinib arm (60 patients vs 48 patients).
Figure 3. ORR in the zanubrutinib arm vs ibrutinib arm*
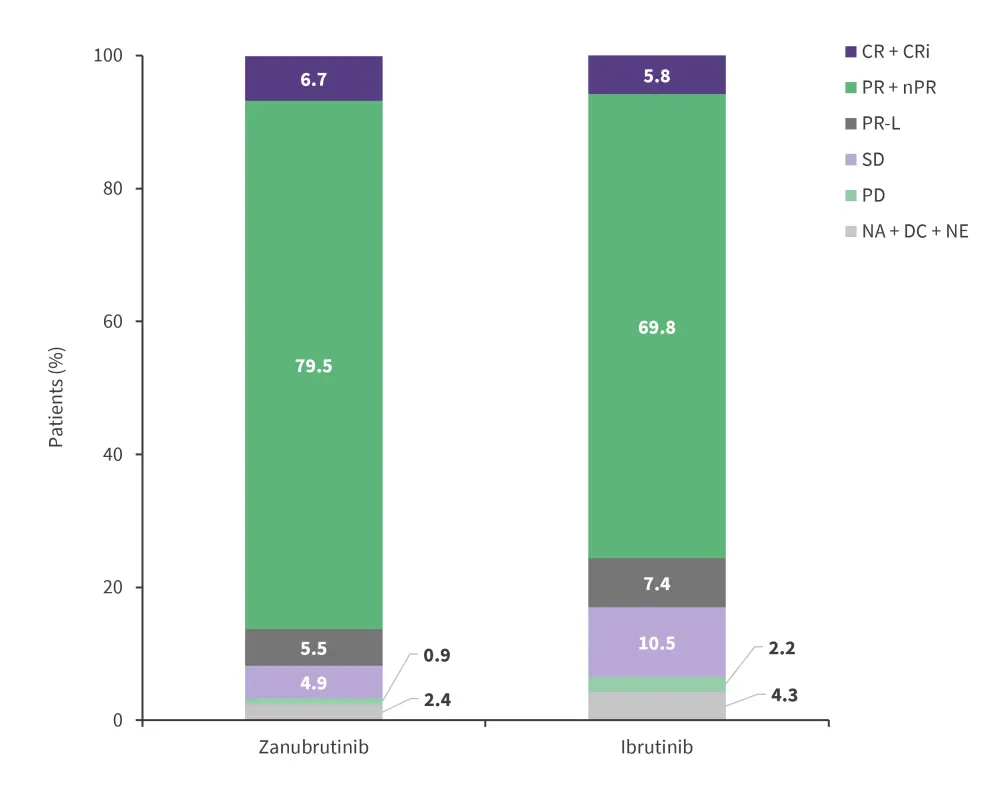
CR, complete response; CRi, complete response with incomplete bone marrow recovery; nPR, nodular partial response; ORR, overall response rate; PD, progressive disease; PR, partial response; PR-L, partial response with lymphocytosis; SD, stable disease.
*Adapted from Brown, et al.2
Safety
- The overall safety profile favored zanubrutinib (Table 2).
- The most common adverse events (AEs) included neutropenia, COVID-19-related AEs, hypertension, upper respiratory tract infection, diarrhea, anemia, and arthralgia
- Numerically lower rates of cardiac AEs were reported in the zanubrutinib arm vs ibrutinib arm
- atrial fibrillation/flutter: 5.2% vs 13.3%, respectively.
- fetal cardiac events: 0 vs 6 patients, respectively.
- serious cardiac AEs: 1.9% vs 7.7%, respectively.
Table 2. Safety and tolerability of the zanubrutinib arm vs ibrutinib arm*
|
AE, adverse event. |
||
|
Characteristic, % (unless otherwise stated) |
Zanubrutinib arm |
Ibrutinib arm |
|---|---|---|
|
Median treatment duration, months |
28.4 |
24.3 |
|
Any grade AE |
98.1 |
99.1 |
|
Grade 3–5 |
67.3 |
70.4 |
|
Grade 5 |
10.2 |
11.1 |
|
Serious AEs |
42.0 |
50.0 |
|
AEs leading to |
||
|
Dose reduction |
12.3 |
17.0 |
|
Dose interruption |
50.0 |
56.8 |
|
Treatment discontinuation |
15.4 |
22.2 |
Conclusion
The presenter concluded that in patients with R/R CLL/SLL, PFS was significantly longer among patients who received zanubrutinib compared with ibrutinib; this was consistent across all subgroups, including the high-risk patients with del(17p)/TP53mut. Zanubrutinib had a favorable safety profile compared with ibrutinib, with a lower rate of treatment discontinuation and fewer cardiac disorder events, including fewer cardiac events leading to death.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


