All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
5-year outcomes of axicabtagene ciloleucel in adults with refractory LBCL: ZUMA-1 trial
CD19-targeted chimeric antigen receptor (CAR) T-cell therapies have significantly advanced the treatment landscape for relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Based on results from the ZUMA-1 trial, axicabtagene ciloleucel (axi-cel) was initially approved for R/R LBCL following ≥2 lines of systemic therapy; it has more recently been indicated for R/R LBCL within 12 months of first-line chemoimmunotherapy, based on outcomes from the ZUMA-7 trial.
The 2-year outcomes of the ZUMA-1 trial (NCT02348216), previously reported on the Lymphoma Hub, demonstrated an 83% objective response rate, 58% complete response (CR) rate, a manageable safety profile, ongoing responses in 39%, and median overall survival (OS) was not reached. Moreover, comparable outcomes to ZUMA-1 have been reported in real-world analyses.
Below, we summarize an article published by Neelapu et al.1 in Blood on the 5-year efficacy and safety outcomes of phase II ZUMA-1 trial, which included an analysis of the durability responses and long-term survival benefit.
Study design
The study design, treatment procedures, eligibility criteria, and baseline and patient characteristics for phase II of the ZUMA-1 study were previously reported on the Lymphoma Hub.
Outcomes
- The primary endpoint was investigator assessed objective response rate according to the International Working Group response criteria for malignant lymphoma.
- Secondary endpoints included duration of response (DOR), progression-free survival (PFS), OS, incidence of select adverse events (AEs) of interest, and blood levels of CAR T cells.
- Exploratory endpoints included event-free survival (EFS), defined as the time from axi-cel infusion until disease progression, initiation of new therapy (excludes hematopoietic stem cell transplantation), or death; time to progression, defined as time from axi-cel infusion until disease progression; time to next therapy, defined as time from infusion until start of new lymphoma therapy; and disease specific survival, defined as the time from infusion until death as a result of progressive disease.
- The correlation between median EFS, key patient/disease characteristics, and the link between DOR and CR achievement at or after Week 4 was also assessed.
Results
Updated efficacy results
Of the 111 patients enrolled in the phase II study, 101 were treated with axi-cel; 84% of these patients had lactate dehydrogenase levels above the upper limit of normal. At the data cut off (August 11, 2021) and a median follow-up of 63.1 months, durable responses were observed (Figure 1). The median DOR was 11.1 months, median duration of CR was 62.2 months, and median duration of PR was 1.9 months. Median DOR was 34.7 months in those with CR by Week 4 and not reached in those who achieved a CR after Week 4.
Figure 1. Response rates*
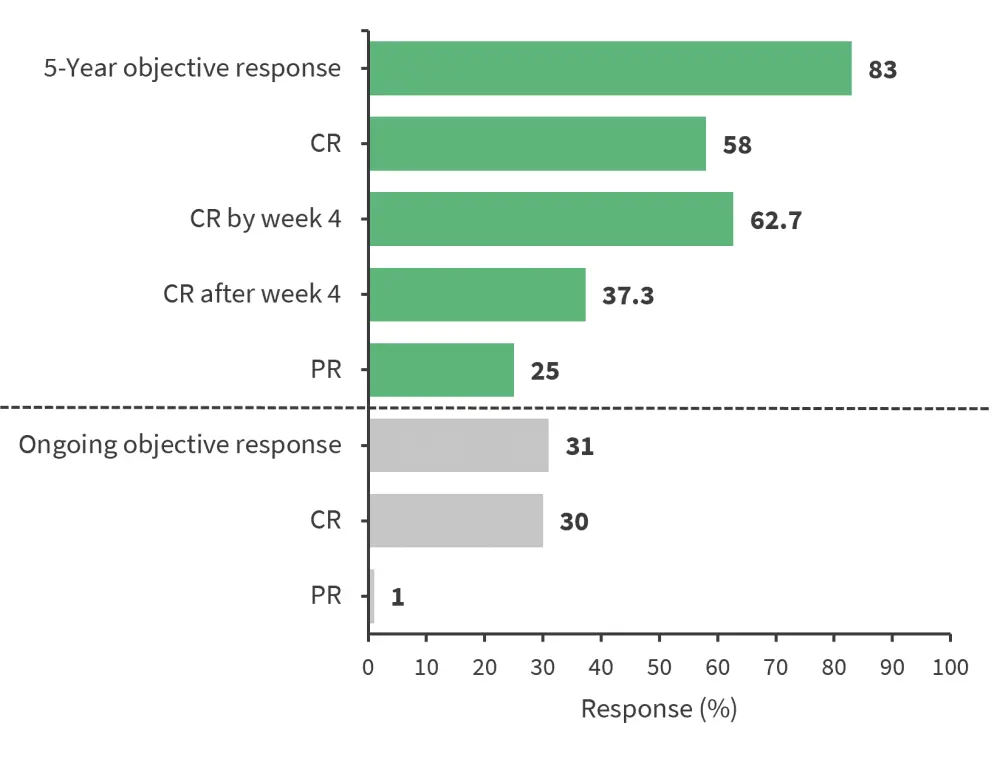
CR, complete response; PR, partial response
*Data from Neelapu, et al.1
The survival outcomes at the data cut off were as follows:
- Median EFS was 5.7 months, with an estimated 5-year EFS of 30.3%.
- A longer median EFS was observed in patients ≥65 years vs <65 years (12.5 months versus 5.6 months) and in those with a tumor burden below the median vs above the median (15.0 months versus 3.1 months); the median EFS outcomes was similar across other subgroups.
- Median PFS was 5.9 months, with an estimated 5-year PFS of 31.8%.
- Median OS was 25.8 months, with an estimated 5-year OS rate of 42.6%; for those who achieved CR, the median OS was not reached with an estimated 5-year OS rate of 64.4%.
- The median time to progression was 6.1 months and the median time to next therapy was 8.7 months.
- A total of 42 patients were alive at the data cut off, including 63% who achieved a CR.
- Among 11 patients who were alive but did not achieve an ongoing response at data cut off, eight received subsequent therapy, with two patients retreated with axi-cel.
- Median disease-specific survival was not reached, with an estimated 5-year rate of 51%.
Updated safety results
At the 5-year follow-up, no new safety signals and no new axi-cel-related serious AEs were reported, with a safety profile comparable to previous analyses.
- Any-grade cytokine release syndrome (CRS) occurred in 94 patients (Grade ≥3 in 11 patients). Neurological events were reported in 65 patients (Grade ≥3 CRS in 30 patients).
- Overall, 43% and 26% of patients received tocilizumab and corticosteroids, respectively, for the management of CRS and/or neurological events.
- Prior to data cut off, the two Grade 3 cytopenias (one anaemia, one neutropenia) previously reported at the 2-year analysis were resolved.
- No axi-cel related secondary malignancies have been reported so far in the study.
- After the 2-year analysis, immunoglobulin therapy was given to three patients (two for prophylaxis and one for Grade 2 axi-cel related immunoglobulin G decreased).
- Overall, 59 deaths have been reported in the study so far, mostly due to progressive disease; the majority of deaths occurred in the first year.
- There have been no deaths due to AEs since the two-year analysis.
Biomarker analysis
Among the biomarker-evaluable patients (n = 97):
- There were higher median peak CAR T-cell levels in blood samples of patients with durable responses at Month 60 post-axi-cel infusion versus those experiencing disease relapse and those not responding (65.76 cells/µL vs 35.27 cells/µL vs 12.08 cells/µL, respectively).
- Higher CAR T-cell levels area under the curve between Days 0 and 28 post infusion was also observed for those with ongoing response vs those experiencing relapse and non-responders.
- As previously reported, B-cell aplasia and recovery were also observed in patients with ongoing response at the 5-year follow-up.
- Among evaluable patients with ongoing responses 3-years post axi-cel infusion, 91% showed polyclonal B-cell recovery and diversity.
Conclusion
This additional follow-up analysis of the ZUMA-1 trial demonstrated the clinical survival benefit and manageable safety profile of axi-cel in patients with refractory LBCL. Moreover, the pharmacokinetic profile showed polyclonal B-cell recovery in a large proportion of patients, with ongoing responses at the 3-year mark, as well as an association between durable responses at the 5-year mark and early CAR T-cells expansion. Overall, these data support the use of axi-cel in aggressive B-cell lymphomas with curative intent.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



