All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
5-year results of the MURANO phase III trial of venetoclax + rituximab vs bendamustine + rituximab in patients with CLL
Bcl2 is overexpressed in chronic lymphocytic leukemia (CLL) and therefore makes an ideal therapeutic target. As a result, in recent years, venetoclax has become a staple treatment for patients with CLL in combination with rituximab. The aim of the MURANO trial (NCT02005471) was to evaluate the combination of venetoclax + rituximab (VenR) against bendamustine + rituximab (BR) in patients with relapsed/refractory (R/R) CLL.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, the 5-year results of the MURANO phase III trial were presented by Arnon Kater.1 This article is based on data presented at the ASH Annual Meeting and Exposition and may supersede the data in the published abstract.
The Lymphoma Hub previously covered the study design and the 4-year results here. A summary diagram of the study design is shown in Figure 1.
Figure 1. MURANO study design1
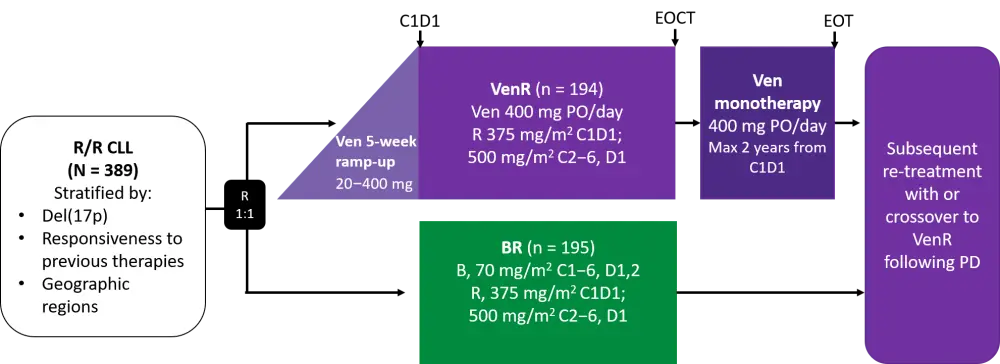
B, bendamustine; BR, bendamustine + rituximab; C, cycle; CLL, chronic lymphocytic leukemia; EOCT, end of combination treatment; EOT, end of treatment; D, day; Del, deletion; PD, disease progression; PO, oral; R/R, relapsed/refractory; R, rituximab; Ven, venetoclax; VenR, venetoclax + rituximab.
Key findings
Survival endpoint results
Progression-free survival (PFS) and overall survival (OS) benefits with VenR over BR were sustained in this extended follow-up. Median PFS for the VenR group (n = 194) was 53.6 months (95% CI, 48.4–57.0), compared with 17.0 months (95% CI, 15.5–21.7) for the BR group (n = 195) (HR, 0.19; 95% CI, 0.15–0.26; p < 0.0001). Whilst 37.8% of patients reached 5-year PFS in the VenR group, 5-year PFS was not evaluable in the BR group.
Median OS was not reached in either treatment arm (HR, 0.40; 95% CI, 0.26–0.62; p < 0.0001). The 5-year OS was 82.1% for VenR and 62.2% for BR.
No new safety signals were recorded in this extended follow-up. There were seven cases of Richter’s transformation in the VenR arm and six in the BR arm. In the VenR arm there was one case of multiple myeloma and one case of acute myeloid leukemia.
Measurable residual disease (MRD)
Undetectable MRD (uMRD; < 10−4) was predictive of improved outcome following the end of treatment (EOT) in the VenR arm, as shown in Table 1. Notably, there was a significantly higher 24-month PFS rate for patients with uMRD compared with the high MRD-positive group (85.4% vs 8.3%, respectively; HR, 0.02; 95% CI, < 0.01–0.18; p < 0.0001).
Table 1. PFS in different MRD categories in the VenR arm following end of treatment1
|
EOT, end of treatment; MRD; measurable residual disease; NE, not estimable; PFS, progression-free survival; uMRD, undetectable MRD; VenR, venetoclax + rituximab. |
|||
|
Category |
n |
PFS (95% CI) since EOT |
|
|---|---|---|---|
|
24 months |
36 months |
||
|
uMRD (< 10−4) |
83 |
85.4% (77.4–93.4) |
61.3% (47.3–75.2) |
|
Low MRD-positive (10−4−10−2) |
23 |
52.2% (31.8–72.6) |
40.7% (19.2–62.2) |
|
High MRD-positive (10−2) |
12 |
8.3% (0.0–24.0) |
NE |
A trend to increased OS at 36 months after EOT was observed for the uMRD group (95.3%; 95% CI, 90.0–100.00) compared with the MRD group (≥ 10-4) (85.0%; 95% CI, 72.8–97.2).
Of the patients that completed 2 years of venetoclax therapy without progressive disease (PD), 64% (83/130) achieved uMRD. For these patients, the median time from EOT to MRD conversion (or PD if it occurred first) was 19.4 months (95% CI, 8.7–28.3). For the 47 MRD-positive patients at this stage, there was a median delay of 25.2 months (95% CI, 19.4–30.4) before clinical PD.
High-risk factors and MRD conversion
The presence of high-risk cytogenetic factors, such as del(17p), genomic complexity (GC; ≥ 3 copy number variations), and unmuted immunoglobulin heavy chain variable region (IGHV), was found to increase the risk of MRD conversion and PD at EOT. All four patients with del(17p) had MRD conversion with subsequent PD, compared with only 22% of patients without this deletion (n = 54). Of the 18 patients with GC, 72% had MRD conversion; 44% converted with subsequent PD, whereas for patients without GC (n = 40), 60% underwent MRD conversion, with 20% developing PD. In the unmutated IGHV group (n = 56), 64% of patients underwent MRD conversion; 37% converted with subsequent PD. By contrast, the mutated IGHV group saw 56% MRD conversion but only 4% with subsequent PD, suggesting slower clonal growth rates.
Clonal growth rate analysis
MRD growth rate from EOT was analyzed in the patient population to better understand the intergroup differences in clinical outcome. Faster clonal growth rates indicated shorter MRD doubling time. Results are shown in Table 2. The VenR group had a longer mean doubling time than the BR group (72 days vs 52 days). Among VenR-treated patients, high-risk features were associated with a shorter MRD doubling time. For patients with del(17p), the doubling time was 45 days, compared with 86 days for patients without del(17p) (the small number of patients with del(17p) must be noted). There was a significantly shorter doubling time for the unmutated IGHV group (60 days) compared with the mutated IGHV group (120 days; p = 0.0057). These results indicate that residual disease may reappear more rapidly in patients with these high-risk factors.
Table 2. MRD doubling time in the overall population and specific high-risk groups1
|
BR, bendamustine + rituximab; del, deletion; GC, genome complexity; IGHV, immunoglobulin chain variable region; MRD, measurable residual disease; mut, mutated; unmut, unmutated; VenR, venetoclax + rituximab. |
|||
|
|
Rate, μ (95% CI) |
Mean doubling time, days (95% CI) |
p value |
|---|---|---|---|
|
Treatment arm |
|||
|
BR (n = 104) |
0.006 (0.0051–0.0065) |
52 (46–59) |
0.0006 |
|
VenR (n = 102) |
0.004 (0.0036–0.0048) |
72 (63–83) |
|
|
High-risk feature |
|||
|
Abnormal del(17p) (n = 4) |
0.0067 (0.0001–0.0083) |
44.9 |
0.5731 |
|
Normal del(17p) (n = 33) |
0.0035 (0.0006–0.014) |
85.6 |
|
|
GC (n = 24) |
0.0049 (0.0001–0.014) |
61.6 |
0.054 |
|
No GC (n = 46) |
0.0031 (0.0006–0.0105) |
96.4 |
|
|
Unmut IGHV (n = 29) |
0.0043 (−0.0015–0.0148) |
60.2 |
0.0057 |
|
Mut IGHV (n = 71) |
0.0022 (−0.0007–0.0096) |
120.3 |
|
Retreatment sub-study
A total of 34 patients who had PD were enrolled in a sub-study in which 24 patients were retreated with VenR and 9 patients crossed over from BR. The median follow-up was 12.1 months. There was an increase in the prevalence of unfavorable cytogenetics within this retreatment group compared to the original study population and their initial response to VenR was suboptimal. Out of 16 patients retreated with VenR and a valid MRD assessment at EOT, there were 14 cases of unmutated IGHV, 10 cases of GC, and 7 cases of abnormal del(17p)/TP53.
Median PFS for the retreated group was lower than for the overall study population (45.7 months, range, 36−58 months) and the median duration between EOT and PD was 23.6 months (range, 10−32 months).
Conclusion
Treatment with VenR produced significant PFS and OS benefits compared with BR in patients with R/R CLL. The majority of patients that completed venetoclax monotherapy achieved uMRD, and MRD was shown to be a strong predictor of outcome. In addition, a long delay between MRD conversion and clinical PD was observed in the patients who achieved uMRD after VenR treatment. High-risk features were associated with faster MRD doubling rates. This extended data supports the use of VenR in patients with R/R CLL, even in patients with high-risk cytogenetic features.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

