All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
A four-factor prognostic model predicts outcome in patients with CLL treated with ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor with clinical efficacy for patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and for those with high-risk untreated disease. Resistance to ibrutinib may develop for some patients who achieve an initial response, which can limit the effectiveness of alternative therapies. Ibrutinib resistance is commonly attributed to prevalence of a B-lymphocyte population carrying BTK and/or PLCG2 gene mutations that reactivate B-cell receptor signaling.
In a report published in the Journal of Clinical Oncology, Inhye Ahn and colleagues describe the development and validation of a prognostic scoring system to predict progression-free survival (PFS) and overall survival (OS) of patients with CLL treated with ibrutinib.1
It is proposed that this four-factor prognostic scoring system could be a new tool for risk stratification and could pave the way for early risk-adapted treatment.
Methods
The study design is summarized in Figure 1. The discovery data set comprised data from 720 patients from five clinical trials, and the validation cohort consisted of 84 patients from a phase II trial at the National Institutes of Health (NIH). All patients received 420 mg/day of ibrutinib. Gene sequencing for the detection of BTK and PLCG2 mutations was also carried out for the NIH study cohort. Results from gene sequencing were used to validate the predictive prognostic index.
Figure 1. Summary of study design and data collection*†
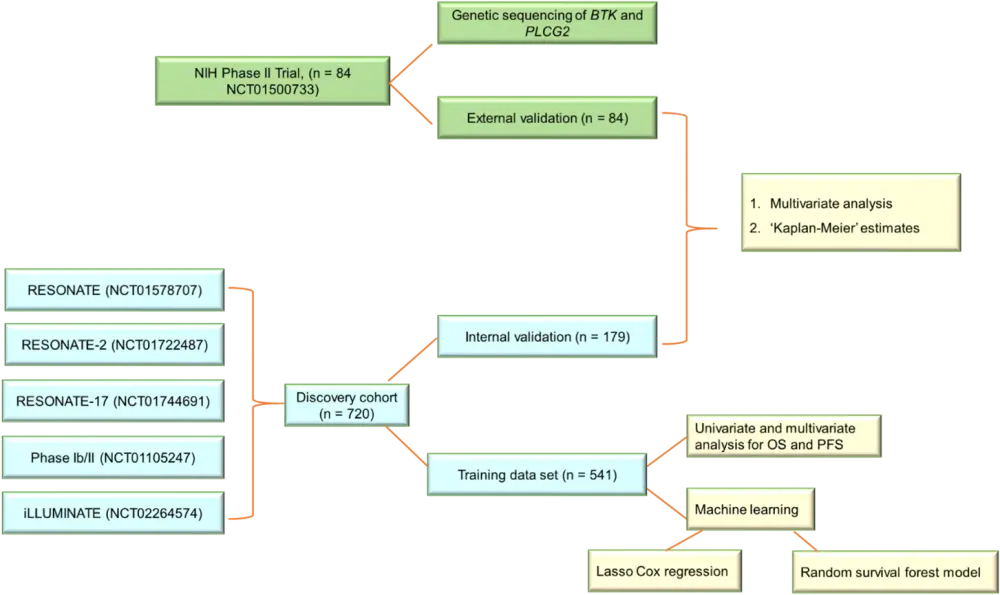
BTK, Bruton’s tyrosine kinase; OS, overall survival; PFS, progression free survival; PLCG2, phospholipase C gamma 2.
*Data from Ahn et al.1
†All statistical analyses were collectively used to create the prognostic model.
Results
In the univariate analysis of the discovery cohort, 11 out of 18 factors tested were associated with inferior OS and PFS (Table 1). Although Rai stage was associated with inferior OS, there was no significant association with PFS. On the other hand, young age, unmutated immunoglobulin heavy chain variable region, and lower lymphocyte count were found to be associated with lower PFS but not OS.
Table 1. Factors associated with inferior OS and PFS by univariate analysis*
|
B2M, β-2 microglobulin; CLL, chronic lymphocytic leukemia; ECOG, Eastern Corporative Oncology Group; HR, hazard ratio; IGHV, immunoglobulin heavy chain variable region; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival. |
||||
|
Factor |
OS |
PFS |
||
|---|---|---|---|---|
|
p value |
HR value |
p value |
HR value |
|
|
ECOG performance status 1–3 |
0.0004 |
1.74 |
0.0013 |
1.50 |
|
Bulky disease ≥5 cm |
0.0003 |
1.73 |
<0.0001 |
1.79 |
|
Hemoglobin <10 g/dL |
0.0002 |
1.78 |
0.0012 |
1.53 |
|
Platelet counts <100 × 109/L |
0.0038 |
1.53 |
0.0091 |
1.38 |
|
Absolute neutrophil count <1.9 × 109/L |
0.0183 |
1.60 |
0.0143 |
1.49 |
|
Relapsed/refractory CLL |
<0.0001 |
2.47 |
<0.0001 |
2.67 |
|
TP53 aberration |
<0.0001 |
2.30 |
<0.0001 |
2.38 |
|
17p deletion |
0.0011 |
2.22 |
0.0001 |
2.12 |
|
B2M ≥5 mg/L |
<0.0001 |
2.28 |
<0.0001 |
2.12 |
|
LDH >250 U/L |
<0.0001 |
1.99 |
<0.0001 |
1.87 |
|
Complex karyotype |
0.0021 |
1.92 |
0.0018 |
1.74 |
In the multivariate analysis, TP53 aberration, R/R CLL, β-2 microglobulin (B2M) ≥5 mg/L, and lactate dehydrogenase (LDH) >250 U/L were consistently associated with inferior PFS and OS in the training, internal validation, and external validation cohorts of ibrutinib-treated patients (Figure 2).
Figure 2. Multivariate analysis for PFS and OS in all cohorts combined, showing HR and p values for TP53 aberration, R/R CLL, high B2M, and high LDH*
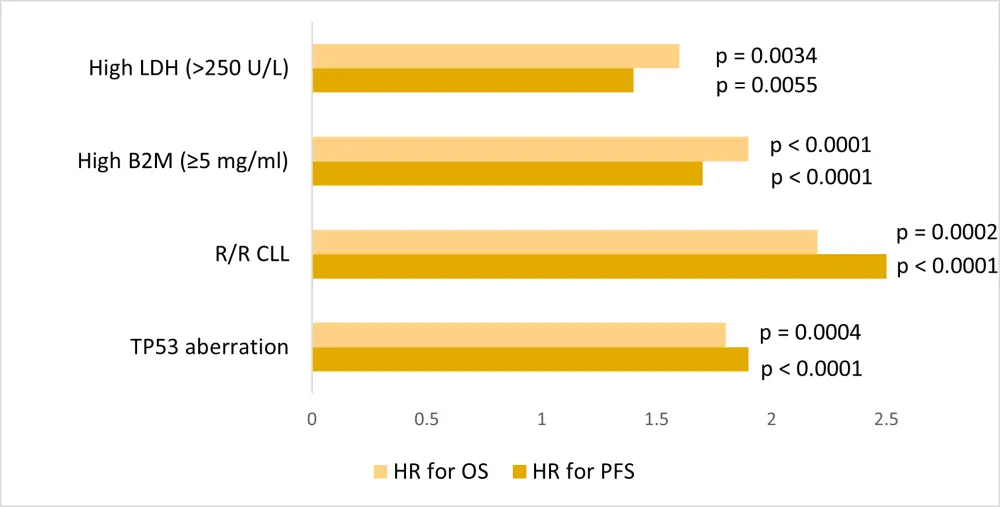
B2M, β-2 microglobulin; CLL, chronic lymphocytic leukemia; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; R/R, relapsed/refractory; TP53, tumor protein 53.
*Data from Ahn et al.1
The four prognostic factors were validated using two machine-learning approaches, lasso Cox regression and random survival forest method, and then used to develop a prognostic index. Three risk groups were identified: low-risk (risk scores 0–1), intermediate (risk score 2), and high-risk (risk scores 3–4). The 3-year PFS and OS rates were significantly different (p < 0.0001) according to risk group for the discovery and external validation cohorts combined (Table 2).
Table 2. PFS and OS rates according to risk group for all cohorts combined
|
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival. |
||||||
|
Risk group |
3-year PFS, % |
HR (95% CI) |
p value* |
3-year OS, % |
HR (95% CI) |
p value* |
|---|---|---|---|---|---|---|
|
Low |
87 |
1 |
— |
93 |
1 |
— |
|
Intermediate (risk score 2) |
74 |
1.9 |
0.0010 |
83 |
2.5 |
0.0002 |
|
High |
47 |
4.7 |
<0.0001 |
63 |
5.3 |
<0.0001 |
The cumulative incidence of BTK and/or PLCG2 mutations was 50%, 40%, and 17% for high-, intermediate- and low-risk groups, respectively. Progression to Richter’s transformation in the respective risk groups was 17%, 5%, and 0%. There was a high correlation between initial time of mutation detection and clinical progression (ρ = 0.93; p = 0.0001). Furthermore, BTK/PLCG2 mutations were almost three times more common in patients with a TP53 aberration (38%), compared with those without the aberration (13%).
Key Points
- Four factors, TP53 aberration, R/R CLL, B2M ≥5 mg/L, and LDH >250 U/L, were independently associated with inferior PFS and OS in patients treated for >5 years with ibrutinib, either as monotherapy or in combination with obinutuzumab.
- A prognostic model consisting of low, intermediate, and high-risk groups was developed using these risk factors. This is contrary to the CLL international prognostic index (CLL-IPI), which categorizes patients into four risk groups, from low to very high.
- The high-risk group had a mortality rate of 53% at 3 years, compared with 13% in the low-risk group.
- Gene sequencing for the detection of BTK and PLCG2 mutations revealed that high-risk patients tended to have a higher mutation frequency compared with the other two groups. However, since these mutations were not detectable at baseline, and were not present in all patients with CLL progression, usefulness as a disease defining biomarker is limited.
- There was a lack of substantial agreement between CLL-IPI and the four-factor model developed in this study (n = 531; weighted κ = 0.45). Hazard ratios were consistently higher for the four-factor model than for CLL-IPI, leading to better discrimination between risk groups for PFS (C‑statistic, 0.69 vs 0.63). Patients in the highest CLL-IPI risk group had a 3-year PFS of 59%, whereas high-risk patients according to the four-factor model had a 3-year PFS of 49%.
Limitations
Limitations to this study, as identified by the authors, included a lack of data for additional important factors, such as CD49d expression and complex karyotype, in some patients. Moreover, since prior treatment was included as an adverse risk factor, there was a variation in survival prediction data for patients with treatment-naïve (TN) CLL and R/R CLL, and almost 30% of patients with TN CLL were excluded from analysis, mostly due to the absence of B2M data. Better estimates of differences in outcome for TN CLL will require a longer follow up of a larger numbers of patients.
Conclusion
The predictive score provided by this new prognostic tool offers an effective way to risk stratify patients with CLL receiving ibrutinib therapy. In clinical practice, this will help to direct patients to risk adapted treatment approaches, and in clinical trials, use of this score can help to stratify patients and determine whether outcomes can be improved for those with the greatest need.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

