All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
AACR 2017 | Immune evasion in Classical Hodgkin Lymphoma
This year’s American Association for Cancer Research (AACR) annual meeting took place on 1–5 April in Washington, DC, USA. The program committee Chair was Kornelia Polyak, MD, PhD, from the Dana-Farber Cancer Institute, Boston, Massachusetts.
On Sunday 2nd April, the “RAOS02 - Immunotherapy of Lymphoid Malignancies” session was introduced by its Chair, Margaret A. Shipp, MD, also from the Dana-Farber Cancer Institute, who gave a talk on immune evasion in Classical Hodgkin Lymphoma (cHL).
The talk began by discussing Reed-Sternberg (RS) cells, which are crippled CD30+ germinal center B-cells that display genomic instability. They are found in primary cHLs with an extensive inflammatory/immune cell infiltrate.
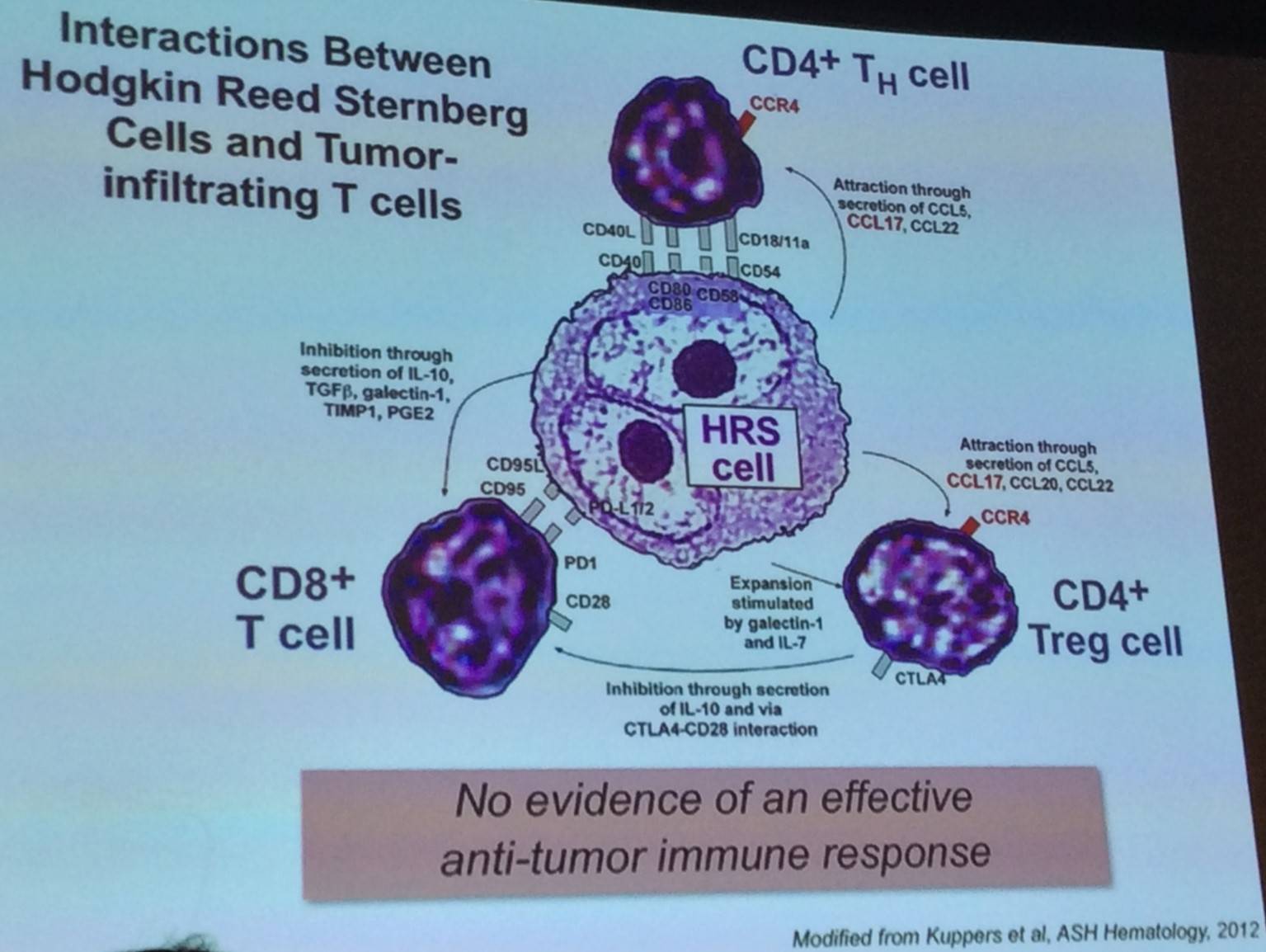
High throughput genomic assays are limited by the unique composition of cHLs. The precise incidence, nature, and prognostic impact of PD-L1/PD-L2 genetic alterations in cHL currently are unclear. The majority of newly diagnosed cHL patients display copy number alterations in PD-L1 and PD-L2, mostly copy gains and amplifications. High level alterations (amplification) can be predictive of outcome – negative standard for therapy. Therefore, does a genetic basis exist for sensitivity to PD-1 blockade?
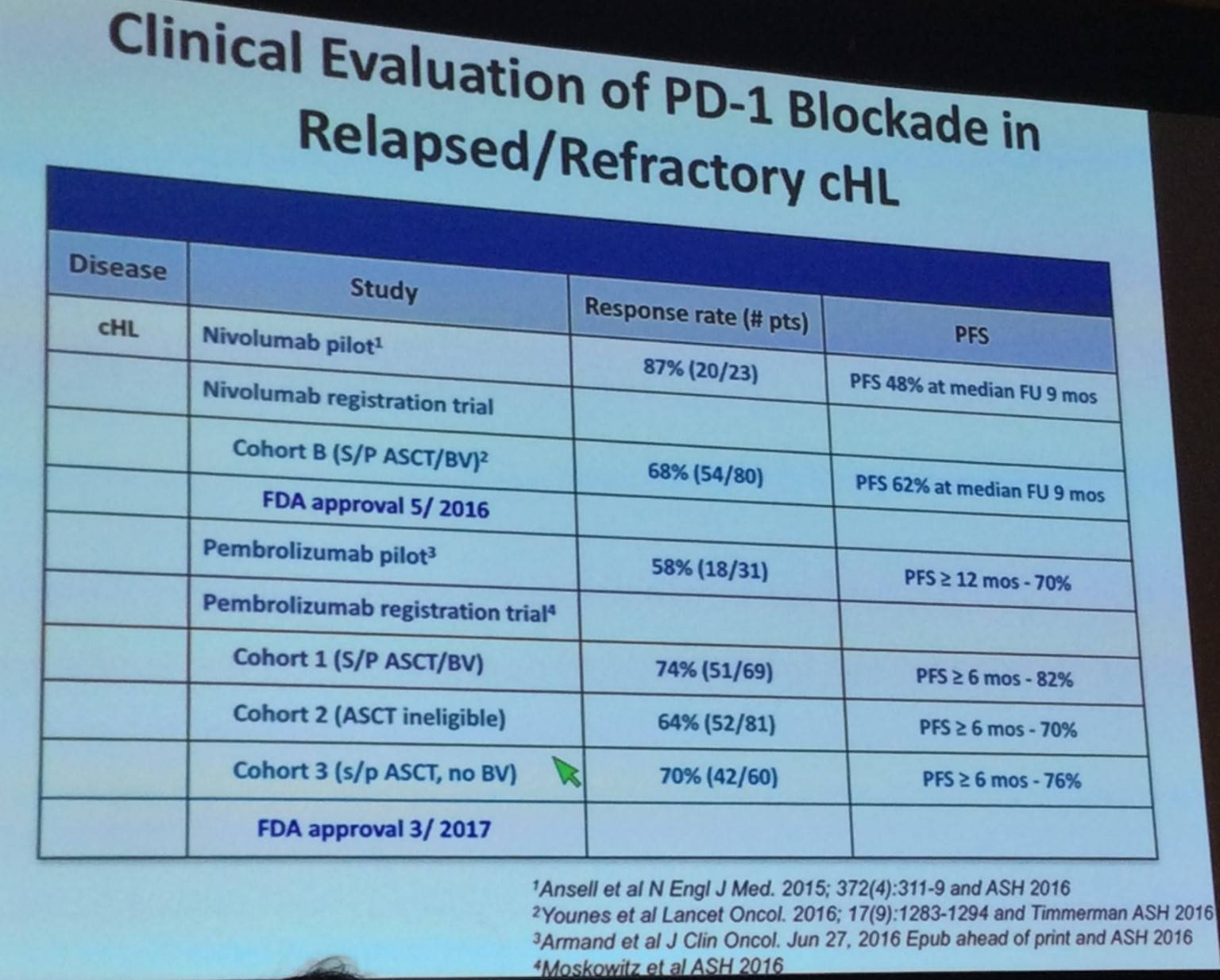
Results of the CA209-205 registration trial indicated that high levels of 9p24.1 alterations and PD-L1 expression are associated with increased sensitivity to PD-1 blockade (Younes et al. Lancet Oncol. 2016).
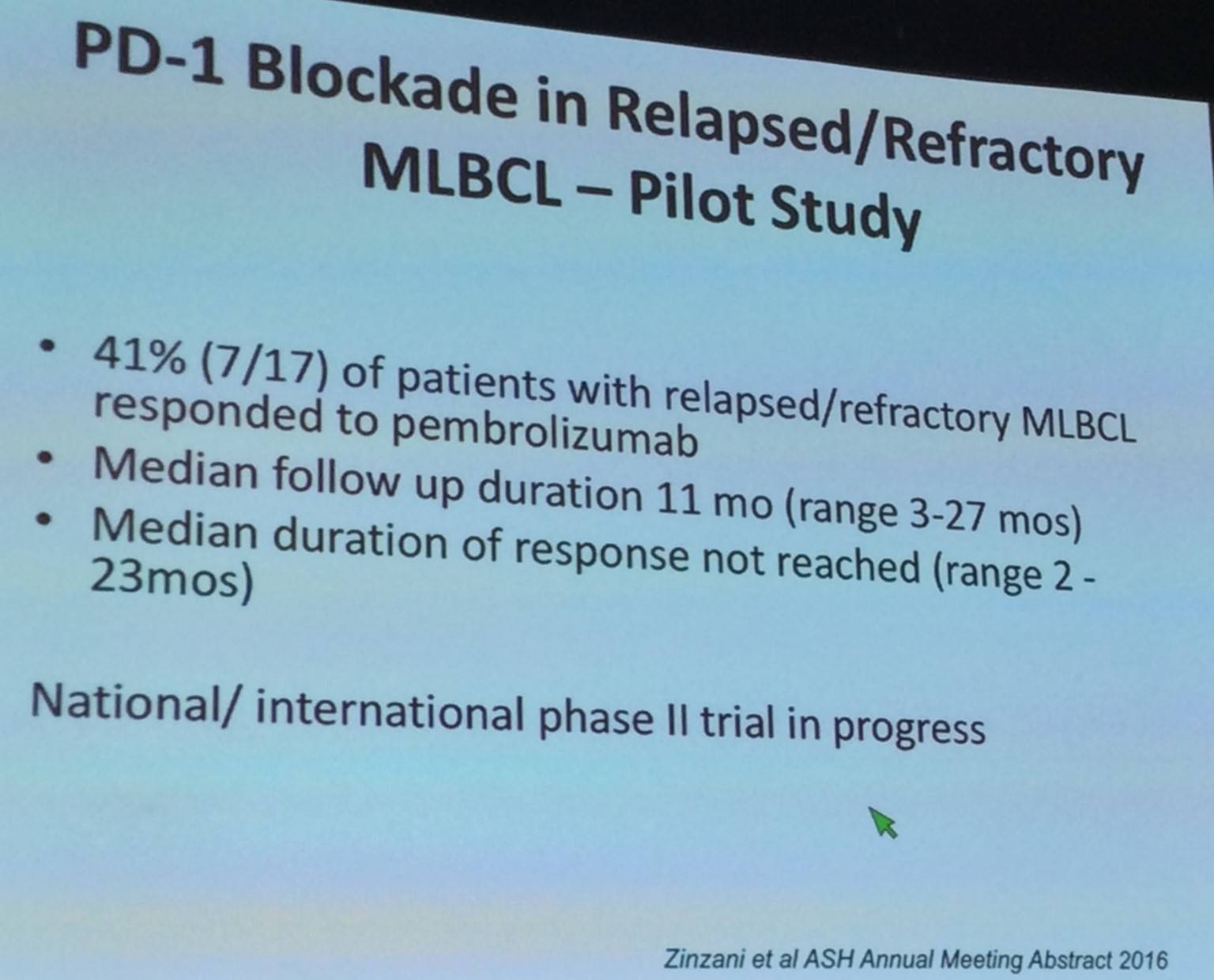
Margaret Shipp also discussed data presented by Zinzani et al. at ASH 2016 of PD-1 blockade in R/R Mediastinal Large B-Cell Lymphoma (MLBCL). 9p24.1 amplification and PD-1L overexpression has been reported in some MLBCL patients (Green et al. Blood. 2010).
The talk then focused on “immune privilege” Lymphomas:
- Take place in sites with limited normal immune responses including CNS and testes
- Large Cell Lymphomas of the CNS and testis
- Low stage (IE) but unfavorable outcome
- Certain shared molecular features
- Proposed inadequate anti-tumor immune response
- Few treatment options available; often ineffective and highly toxic
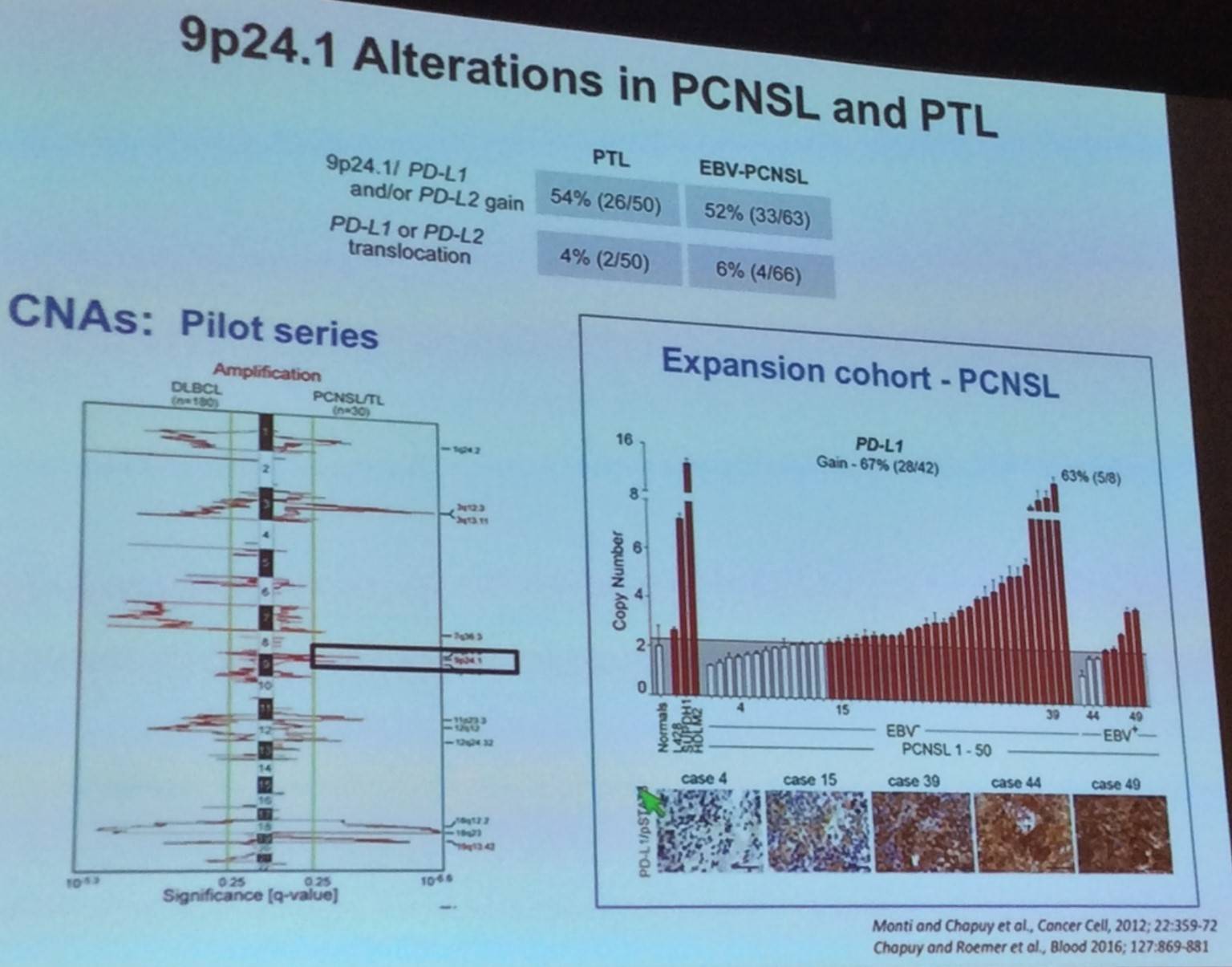
Margaret Shipp outlined findings reported by Nayak et al. during ASH 2016, exploring the use of nivolumab in Primary Central Nervous System Lymphoma (PCNSL) and Primary Testicular Lymphoma (PTL):
- R/R PCNSL (n=4), CNS relapse of PTCL (n=1)
- Clinical and radiographic responses to PD-1 blockade = 100% (5/5): 4 CRs and 1 PR
- Three patients are Progression Free (PF) at 13–17+ months; 2 patients PF at 14 and 17 months
- The CA209-746 national/international trial of nivolumab in R/R PCNSL and PTL is underway
Following this the ways in which PD-1L overexpression occurs in lymphoid malignancies was discussed:
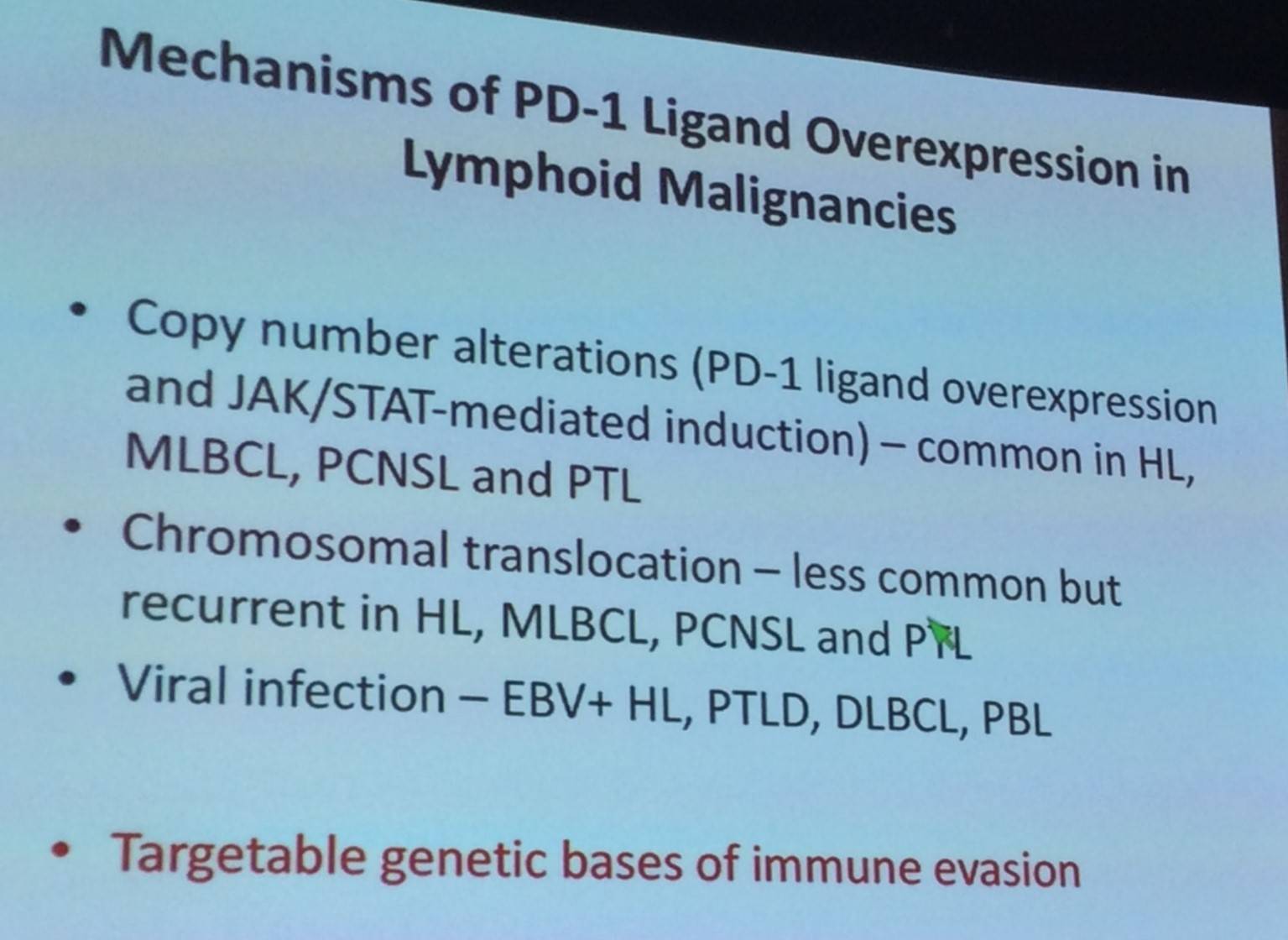
Margaret Shipp discussed findings published by Roemer et al. in Cancer Immunology Research in 2016, exploring the relationship between expression of β2M, MHC class I, and MHC class II, and 9p24.1 aberrations.
- Reduced/missing β2M/MHC class I: 85/108 (79%)
- Reduced/missing MHC class II: 72/108 (67%)
- No association found between β2M, MHC class I, and MHC class II expression and 9p24.1 genetic alterations
The talk was concluded by stating that this lack of β2M/MHC class I expression in RS cells in cHL has caused speculation regarding alternate mechanisms of PD-1 blockade in cHL.
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

