All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Incyte, and Lilly. The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
AACR 2017 | Understanding success and failure of CAR T-cell therapy for Non-Hodgkin Lymphoma
This year’s American Association for Cancer Research (AACR) annual meeting took place on 1–5 April in Washington, DC, USA. The program committee Chair was Kornelia Polyak, MD, PhD, from the Dana-Farber Cancer Institute, Boston, Massachusetts.
On Sunday 2nd April, during the “RAOS02 - Immunotherapy of Lymphoid Malignancies” session, Stanley R. Riddell of the Fred Hutchinson Cancer Research Center, Seattle, WA, gave a talk titled: “Understanding success and failure of T-cell therapy for B-cell malignancies.”
Stanley Riddell began his talk by outlining the advantages and disadvantages of Chimeric Antigen Receptors (CARs) as well as the potential targets for CAR directed therapy in a range of B-cell malignancies:
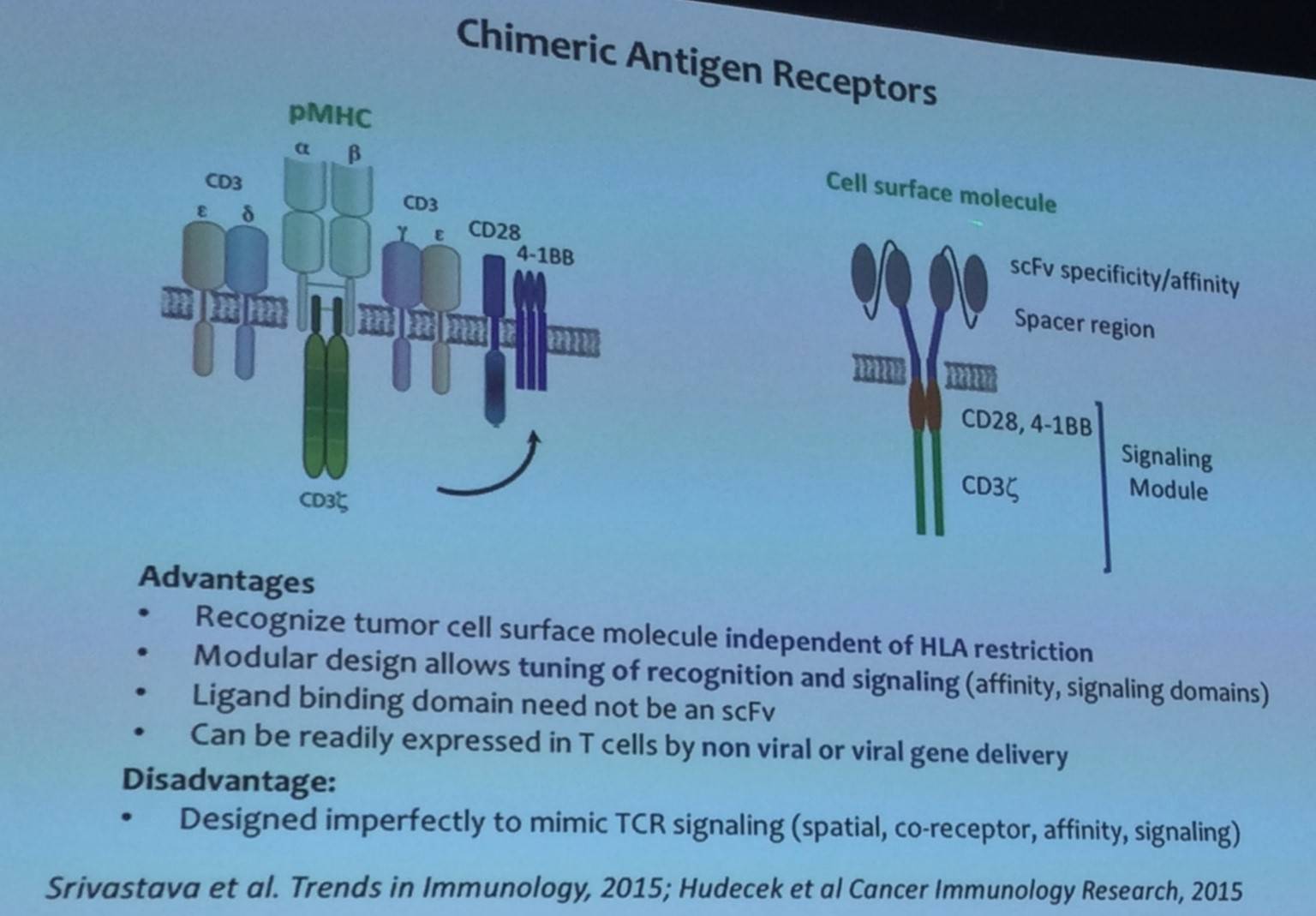


Moreover, patients suffering with B-cell malignancies are often lymphopenic, and harbor a variable distribution of phenotypic subsets. Ideally, effective T-cell subsets need to be identified and consistent products need to be produced for all patients. However, currently a method for accurate and quick selection of T-cell subsets with clinical grade selection agents is not available.
The talk then focused on data published by Turtle et al. in Science Translational Medicine in 2016, who treated NHL patients with CAR T-cells prepared from a strictly defined ratio of CD4 to CD8 T-cells.

It has been found that different subtypes of NHL respond differently to CAR T-cell therapy. Stanley Riddell then asked what mediates resistance to CAR T-cells in some patients, providing three potential mechanisms:
- Local resistance to CAR T-cells infiltrating in tumor microenvironment
- Immune checkpoint molecules on tumor (e.g. PD-L1) and CAR T-cells (PD-1, Lag-3, Tim3)
- Local metabolic impediments to CAR T-cell function
The talk then focused on CAR T-cell therapy for CLL patients, prior to ibrutinib, and has resulted in high response rates. In 24 patients (n = 19 ibrutinib refractory; n = 3 ibrutinib intolerant), ORR was 74% (14/19), CR by iwCLL criteria was 21% (4/19) but CR by PET was 64% (7/11).
Stanley Riddell also presented incidence data of cytokine release syndrome and neurotoxicity that occurs as a result of CAR T-cell therapy:

It has also been found that high levels of IL-15 and IL-6, as well as low levels of TGF-β, on day 1 are associated with grade 3 or higher neurotoxicity.
To counteract B-cell aplasia caused by persistence of CAR T-cells, it has been suggested that antibody targeting of EGFRt can eliminate CD19 CAR T-cells allowing B-cells to recover.
Stanley Riddell concluded the talk with a succinct summary slide:

References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

