All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
AACR 2017 | Poster 4079/19 – first-in-class SH7139: pre-clinical toxicology and safety in Non-Hodgkin Lymphoma
At the American Association for Cancer Research (AACR) annual meeting in Washington, DC, USA, on Tuesday 4th April, a poster session titled “Mechanistic Understanding of Novel Anticancer Therapies” took place.
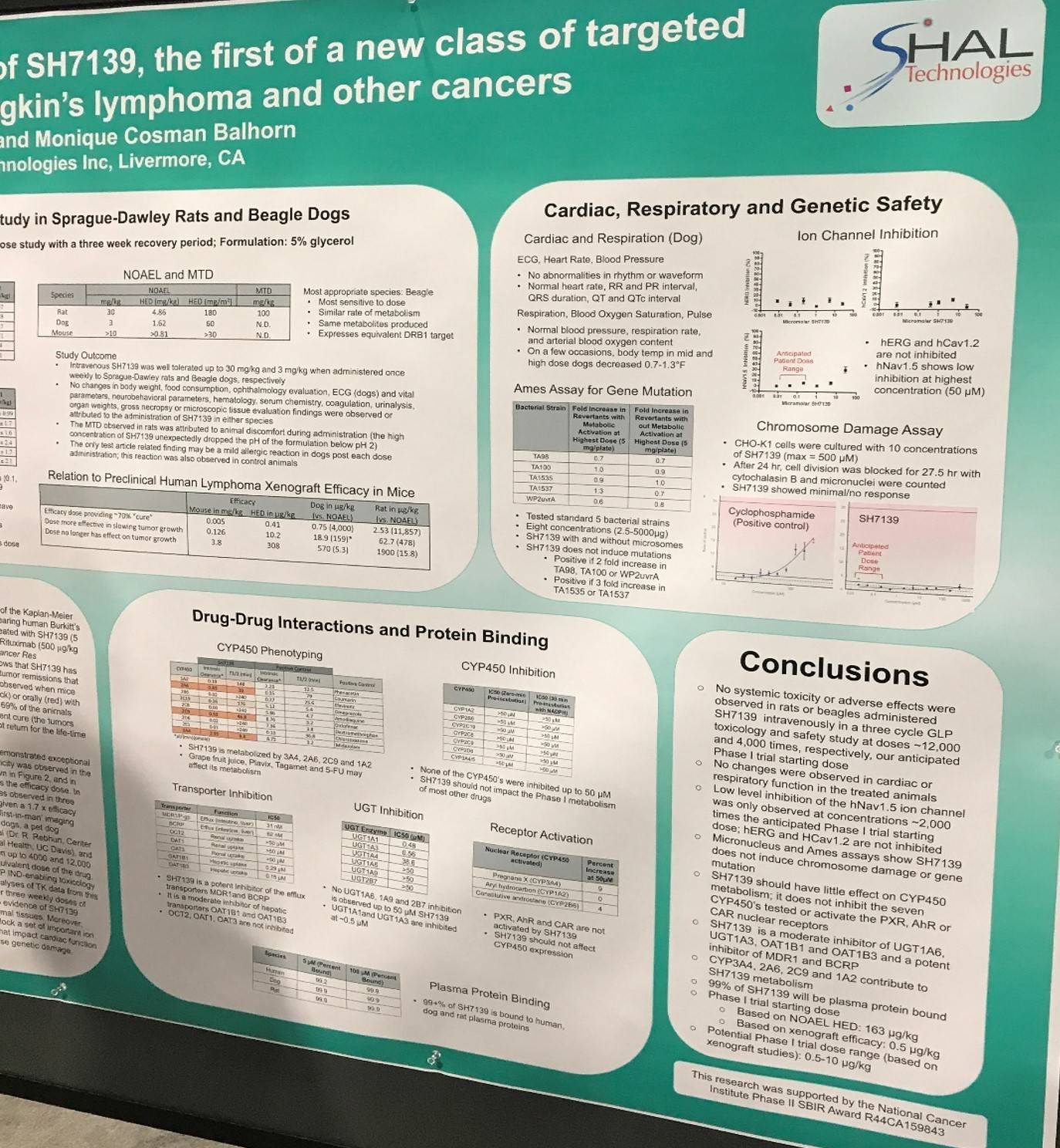
One of the posters on display (4079 / 19) was titled “Pre-clinical toxicology and safety of SH7139: The first of a new class of targeted therapeutics for non-Hodgkin's lymphoma and other cancers” by Rod Balhorn and Monique Cosman Balhorn of SHAL Technologies Inc., Livermore, CA.
SH7139 is a Selective High Affinity Ligand (SHAL) and functions similarly to an antibody-drug conjugate and a pro-drug. It targets and binds a unique structural epitope within the HLA-DR10 antigen-binding pocket. HLA-DRs containing this epitope are expressed in approximately 85% of B-Cell Lymphomas. Upon binding, SH7139 is internalized into the cytoplasm where it is concentrated and metabolized, releasing toxic metabolites that inhibit a range of cellular activities needed for tumor cell function.
The group carried out acute dose range finding (IV bolus) and multiple dose (IV bolus on days 1, 8, and 15) toxicology and safety studies with SH7139 in rats and dogs.
Key Highlights:
- In the rat studies, No Observed Adverse Effect Level (NOAEL) = 30mg/kg; 11,854-fold higher than efficacy dose
- Beagle dogs were found to be the most sensitive species (NOAEL = 0.3mg/kg, 395-fold higher than efficacy dose); doses at 1 and 10mg/kg produced a reversible allergic-type reaction
- In rats, a Maximum Tolerated Dose (MTD) of 100mg/kg was reached
- No attempt was made to identify the MDT in dogs
- In vitro assays carried out to assess potential cardiac safety showed SH7139 did not affect potassium (hERG) and calcium (hCaV1.2) ion channel function up to the highest concentration tested (25μM)
- A detectable effect above background was reported with the hNaV1.5 sodium channel at 25μM, a plasma concentration that would only be reached in patients given a dose ~2,000-fold higher than efficacy dose
- SH7139 did not induce gene mutations in five tester strains of Salmonella and E. coli (AMES assay)
- In a CHO-K1 micronucleus assay, no genotoxicity reported in the presence of S9 over the entire concentration range tested
- In the absence of S9, a positive response (3.3 fold) just above the triggering threshold (3 fold) of the assay was observed at 500μM SH7139 (the highest concentration)
The poster was concluded by stating that based on these results and others reported from murine xenograft efficacy studies, a maximum recommended phase I trial starting dose has been cautiously set at 0.5μg/kg with a safety factor of 324.
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

