All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
AACR 2020 | The efficacy and safety of geptanolimab (GB226) in patients with R/R PTCL
Peripheral T-cell lymphoma (PTCL) is a heterogeneous disease associated with poor prognosis. In Western countries, PTCL accounts for 5–10% of non-Hodgkin lymphomas, with a higher incidence in China (25–30%).1 Despite the recent development of several novel agents, including anti-CD30 immunoconjugate, antifolate and histone deacetylase inhibitors, patients with relapsed or refractory (R/R) disease experience modest improvement in clinical outcomes. Therefore, the need for a more active treatment remains.
One of the features of PTCL is overexpression of programmed death-ligand 1 (PD-L1). Yuankai Shi and colleagues explored whether targeting PD-L1 with a monoclonal antibody against PD-1, geptanolimab (GB226), could improve outcomes of patients with R/R PTCL. The efficacy and safety results of this multicenter, open-label, single-arm, phase II trial (NCT03502629) were presented during the American Association for Cancer Research (AACR) Virtual Annual Meeting I 2020.1
Study design
- In total, 102 patients were enrolled in the study between July 8, 2018, and August 2019
- Eligibility criteria included
- Age ≥ 18 years
- Histological diagnosis of PTCL
- R/R PTCL after systematic treatment
- Eastern Cooperative Oncology Group (ECOG) score 0–1
- Patients received an intravenous infusion of geptanolimab 3 mg/kg once every two weeks until disease progression, death, unacceptable toxicity or withdrawal of consent
- Study endpoints:
- Primary
-
-
- Objective response rate (ORR) by the independent review committee (IRC) according to Lugano 2014 criteria
-
-
- Secondary
-
-
- Duration of response (DOR)
- Disease control rate (DCR)
- Progression-free survival (PFS)
- Overall survival (OS)
- Time to response (TTR)
- Safety
- Immunogenicity
- Exploratory
- Correlative biomarker analysis
-
Results
- The date for data cut off was November 1, 2019
- Out of 102 patients who received treatment
- 26 patients continued with treatment
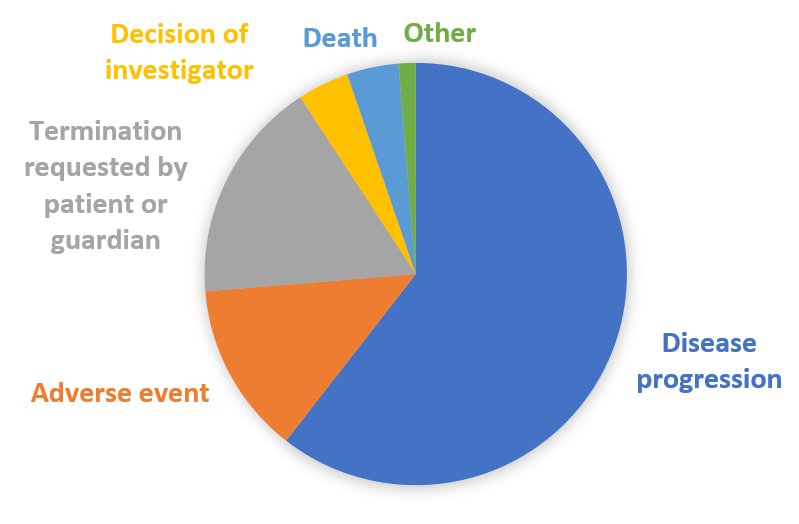
- 76 patients were withdrawn from treatment. Reasons for continuation are presented in Figure 1
Figure 1. Reasons for treatment discontinuation.
Patient characteristics
Patients and disease characteristics are presented in Table 1
Table 1. Baseline patient and disease characteristics Characteristics Geptanolimab (N = 102) Median age (range), years 52.5 (18–78) Male gender, % 68.6
Prior lines of treatment, %
1
2
≥ 3
41.2
33.3
25.5
Stage of disease, %
I–II
III–IV
16.7
82.4
PTCL subtype, %
PTCL-NOS
ALCL ALK+
ALCL ALK-
ENKTL
Other
38.2
6.9
11.8
21.6
21.6
ALCL, anaplastic large cell lymphoma; ENKTL, extranodal NK/T-cell lymphoma nasal type; NK, natural killer; PTCL, peripheral T-cell lymphoma; PTCL-NOS, PTCL-not otherwise specified
Efficacy
- Based on IRC assessment disease control rate (DCR) was 55.9% and ORR was 36.3% (Overall efficacy is presented in Table 2)
- The highest DCR was seen in patients with anaplastic large cell lymphoma (ALCL) ALK+, extranodal NK/T-cell lymphoma nasal type (ENKTL), and ALK- (71.4%, 68.2%, and 66.7%, respectively)
- The highest ORR was seen in patients with ALCL ALK- and ALK+, as well as ENKTL (58.3%, 42.9% and 40.9%, respectively)
- Median PFS by IRC evaluation was 2.69 (1.74–4.21) months
- PFS rate at 3 months was 44.1% (95% CI, 33.8–53.9)
- PFS rate at 6 months was 38.6% (95% CI, 28.5–48.7)
- Median overall survival by IRC evaluation was not reached (NR, 8.15 months–NR)
Table 2. Overall geptanolimab efficacy at data cut off
|
Efficacy |
Investigator assessed (N = 102) |
IRC assessed (N = 102) |
|
Best overall response, % CR PR SD PD Unable to evaluate Not evaluated |
6.9 28.4 18.6 35.2 1.0 9.8 |
10.8 25.5 19.6 33.3 0 10.8 |
|
ORR (95% CI), %* All subsets PTCL-NOS ENKTL ALCL ALK+ ALCL ALK- Others |
35.3 (26.09–45.38) NA NA NA NA NA |
36.3 (26.96–46.39) 28.2 40.9 42.9 58.3 31.8 |
|
DCR (95% CI), %† All subsets PTCL-NOS ENKTL ALCL ALK+ ALCL ALK- Others |
53.9 (43.77–63.84) NA NA NA NA NA |
55.9 (45.71–65.71) 46.2 68.2 71.4 66.7 50.0 |
|
Medium DOR (95% CI), months |
4.14 (1.45–NR) |
6.83 (5.13–NR) |
|
Medium TTR (95% CI), months |
2.79 (2.66–5.65) |
4.04 (1.48–8.25) |
|
ALCL, anaplastic large cell lymphoma; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; ENKTL, extranodal NK/T-cell lymphoma nasal type; IRC, independent review committee; NA, not assessed; NR, not reached; ORR, objective response rate; PD, progressive disease; PR, partial response; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; SD, stable disease; TTR, time to response *defined as CR + PR †defined as CR + PR + SD |
||
- Subgroup analysis revealed:
- Patients with ≤ 2 previous lines of therapy compared to those with > 2 had higher DCR (59.2% vs 46.2%) and median PFS (2.9 vs 1.4 months)
- There was no impact on ORR
- Patients with previous exposure to chidamide (a new histone deacetylase inhibitor approved in China for treatment of PTCL after front-line failure) compared to those without had lower DCR (45.8% vs 59.0%) and longer median PFS (4 vs 2.7 months)
- There was no impact ORR
- Patients with a history of ASCT had higher ORR (57.1%) compared those without (34.7%)
- There was no impact on DCR or PFS
- Higher PD-L1 positivity score (≥ 50 vs <50) correlated with enhanced
- ORR (46.0% vs 24.4%)
- DCR (64.0% vs 43.9%)
- Median PFS (6.2 vs 1.5 months)
- Median DOR (NR vs 6.8 months)
- Patients with ≤ 2 previous lines of therapy compared to those with > 2 had higher DCR (59.2% vs 46.2%) and median PFS (2.9 vs 1.4 months)
Safety
- Treatment-emergent adverse events (TEAEs) of any Grade were experienced by 92.2% of patients, including Grade ≥ 3 by 55.9% of patients
- TEAEs Grade ≥ 3 were reported in 23.5% of patients, with lymphocytopenia, thrombocytopenia, and anemia being the most common (3.9%, 3.9%, and 2%, respectively
- Serious adverse events (SAEs) were experienced by 38.2% of patients,
- 15.7% of SAEs were treatment-related
- Immune-related AEs were present in 35.3% of patients, including 10.8% with Grade ≥ 3, with pulmonary infection, pulmonary inflammation skin rash, and pruritus the most common Grade ≥ 3 (2%, 1%, 1%, and 1%, respectively)
- TEAEs led to treatment discontinuation in 20.6% of patients and death in 4.9% of patients
Conclusion
The results of the study demonstrate that geptanolimab has promising clinical activity in patients with R/R PTCL. The benefit was seen across different subtypes, with ORR especially high in ALCL and ENKTL. The correlation of response with the level of PD-L1 expression suggests that this could be used to select patients most likely to respond. The safety profile was manageable. However, further studies evaluating efficacy and safety are needed.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

