All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Anti-CD30 CAR T-cell therapy in patients with heavily pretreated R/R HL
Patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL) whose salvage therapy has failed have a poor prognosis. Treatment with chimeric antigen receptor (CAR) T-cell therapy, which has shown effectiveness in B-cell malignancies, could be a valid alternative for HL treatment.
A phase I study, evaluating the safety of anti-CD30 CAR T-cell therapy in patients with HL, reported that the infusion of CD30-targeting CAR T cells (CD30.CAR-Ts) with no previous lymphodepleting chemotherapy was well tolerated, but resulted in only a 33% overall response rate (ORR).1 Two parallel phase I/II trials have evaluated the efficacy and safety of the autologous CD30.CAR-Ts after lymphodepleting chemotherapy in heavily pretreated patients with R/R HL. The results of these studies were recently published by Carlos Ramos and colleagues in the Journal of Clinical Oncology.2
Study design2
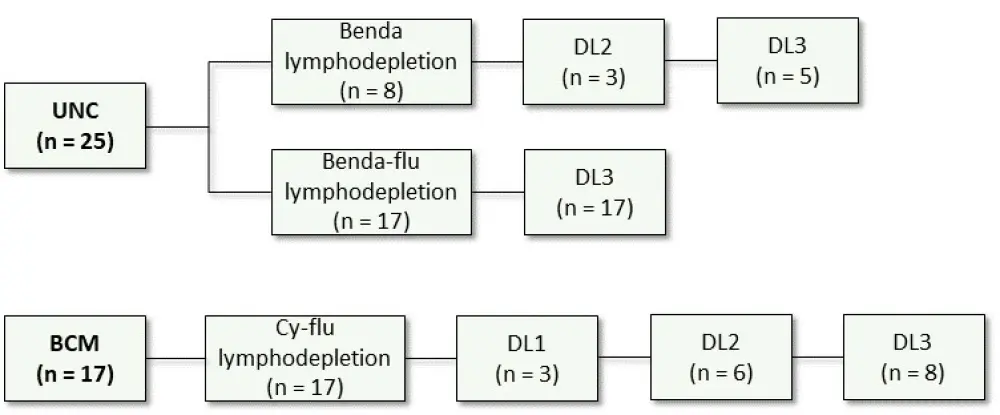
The two parallel phase I/II trials enrolled patients with R/R CD30+ HL who relapsed after ≥ 2 lines of therapy. Patients were enrolled at two centers: the University of North Carolina (UNC, NCT02690545; n = 28) and the Baylor College of Medicine (BCM, NCT02917083; n = 28). Bridging chemotherapy was allowed before lymphodepletion. Of the 28 patients enrolled at UNC, 25 were treated. Of the 28 patients enrolled at BCM, 17 were treated, including one patient that was previously treated at UNC.
Treatment schedule
- Lymphodepletion
- At UNC: Bendamustine (benda) 90mg/m2/day for two days for the first cohort (n = 8), and benda 70 mg/m2/day plus fludarabine (flu) 30 mg/m2/day for 3 days for the second cohort.
- At BCM: Cyclophosphamide (Cy) 500 mg/m2/day and flu 30 mg/m2/day for 3 days.
- Patients received CD30.CAR-T infusion 2–5 days after lymphodepletion:
- At BCM, patients received either 2 × 107 CAR T cells/m2 (dose level [DL] 1), 1 × 108 CAR T cells/m2 (DL2), or 2 × 108 CAR T cells/m2 (DL3).
- At UNC, patients received 1 × 108 CAR T cells/m2 (DL 2) or 2 × 108 CAR T cells/m2 (DL3).
- In patients with stable disease (SD) or partial response (PR) after the first treatment, a second infusion was allowed.
The treatment scheme is reported in Figure 1.
Figure 1. Treatment scheme2
Endpoints
The primary endpoint for both studies was safety. Secondary endpoints were ORR, overall survival (OS), and expansion and persistence of CD30.CAR-Ts in the peripheral blood after infusion.
Results2
Patient characteristics
Baseline patient characteristics are reported in Table 1.
Table 1. Baseline patient characteristics2
|
allo-SCT, allogeneic stem cell transplantation; auto-SCT, autologous stem cell transplantation; benda, bendamustine; BV, brentuximab vedotin; CPI, checkpoint inhibitor; Cy, cyclophosphamide; ECOG PS, Eastern Cooperative Oncology Group performance status; flu, fludarabine; HL, Hodgkin lymphoma; MC, mixed cellularity; NOS, not otherwise specified; NS, nodular sclerosis. *Both treatments are included for the patient who was treated twice. |
||||
|
Characteristic |
All patients (N = 42)* |
Benda (n = 8)* |
Benda-flu (n = 17) |
Cy-flu (n = 17)* |
|---|---|---|---|---|
|
Median age, years (range) |
35 (17─69) |
49 (23─67) |
32 (23─45) |
36 (17─69) |
|
HL subtype, n (%) |
|
|
|
|
|
NS |
32 (76) |
6 (75) |
10 (59) |
16 (94) |
|
MC |
4 (10) |
2 (25) |
2 (12) |
0 (0) |
|
NOS |
6 (14) |
0 (0) |
5 (29) |
1 (6) |
|
Stage at disease, n (%) |
|
|
|
|
|
I─II |
14 (33) |
1 (13) |
7 (41) |
6 (35) |
|
III─IV |
28 (67) |
7 (88) |
10 (59) |
11 (65) |
|
ECOG PS ≥ 1, n (%) |
34 (81) |
5 (63) |
12 (71) |
17 (100) |
|
Median number of prior therapies (range) |
7 (2─23) |
7.5 (5─17) |
8 (3─23) |
5 (2─10) |
|
Bridging therapy, n (%) |
28 (67) |
8 (100) |
10 (59) |
10 (59) |
|
Prior BV, n (%) |
38 (90) |
8 (100) |
16 (94) |
14 (82) |
|
Prior CPI, n (%) |
34 (81) |
7 (88) |
13 (76) |
14 (82) |
|
Prior auto-SCT, n (%) |
32 (76) |
7 (88) |
14 (82) |
11 (65) |
|
Prior allo-SCT, n (%) |
10 (24) |
2 (25) |
8 (47) |
0 (0) |
Safety
The treatment was well tolerated, with no dose-limiting toxicities associated with CD30.CAR-T infusions observed. The most common ≥ Grade 3 adverse events are reported in Table 2.
Table 2. Most common Grade 3 or higher adverse events2
|
Benda, bendamustine; CRS, cytokine release syndrome; Cy, cyclophosphamide; flu, fludarabine. *Both treatments are included for the patient who was treated twice. |
||||
|
Adverse event, n (%) |
All patients (N = 42)* |
Benda (n = 8)* |
Benda-flu (n = 17) |
Cy-flu (n = 17)* |
|---|---|---|---|---|
|
Lymphopenia |
42 (100) |
8 (100) |
17 (100) |
17 (100) |
|
Leukopenia |
24 (57) |
3 (38) |
8 (47) |
13 (76) |
|
Rash (any grade) |
20 (48) |
2 (25) |
4 (24) |
14 (82) |
|
Neutropenia |
20 (48) |
2 (25) |
7 (41) |
11 (65) |
|
Thrombocytopenia |
11 (26) |
1 (13) |
7 (41) |
3 (18) |
|
Grade 3–4 thrombocytopenia not resolved by Day 28 |
10 (24) |
0 (0) |
7 (41) |
3 (18) |
|
CRS (all Grade 1) |
10 (24) |
1 (13) |
2 (12) |
7 (41) |
In total, cytokine release syndrome (CRS) was observed in 24% of patients, all of which were Grade 1 with a median onset of 10 days (range, 7─24) and a median duration of 4 days (range, 1─6). All the CRS events resolved spontaneously without the need for tocilizumab or corticosteroids.
Neurologic toxicity was not observed.
Efficacy
Clinical responses were evaluated in 37 patients with measurable disease at the time of treatment, and rates are reported in Table 3. In patients who received flu-based lymphodepletion, the ORR was 72% (n = 32), with 59% of patients (n = 19) achieving a complete response (CR).
Table 3. Clinical responses2
|
Benda, bendamustine; CR, complete response; Cy, cyclophosphamide; flu, fludarabine; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease. |
||||
|
Response |
All patients (n = 37) |
Benda (n = 5) |
Benda-flu (n = 15) |
Cy-flu (n = 17) |
|---|---|---|---|---|
|
ORR, n (%) |
|
|
|
|
|
CR + PR |
23 (62) |
0 (0) |
12 (80) |
11 (65) |
|
Response rate, n (%) |
|
|
|
|
|
CR |
19 (51) |
0 (0) |
11 (73) |
8 (47) |
|
PR |
4 (11) |
0 (0) |
1 (7) |
3 (18) |
|
SD |
4 (11) |
1 (20) |
1 (7) |
2 (11) |
|
PD |
10 (27) |
4 (80) |
2 (13) |
4 (24) |
After a median follow-up of 533 days:
- 1-year progression-free survival (PFS) in 37 evaluable patients was 36% (95% CI, 21─51), with longer PFS in patients receiving a flu-based conditioning than in patients receiving benda alone (p = 0.0002).
- Median PFS for the 19 patients who achieved CR was 444 days.
- 1-year OS for all 41 patients (counting the one patient treated at both sites only once) was 94% (95% CI, 79─99).
CAR-T cell expansion and persistence
In patients receiving flu-based lymphodepletion, the peak of CD30.CAR-T expansion was observed in the first 2─3 weeks after infusion.
CD30.CAR-Ts persistence was cell-dose dependent, with a higher persistence in patients receiving 2 × 108 CAR-Ts/m2 than in patients receiving 2 × 107 CAR-Ts/m2 or 1 × 108 CAR-Ts/m2 (p < 0.001), irrespective of the lymphodepleting regimen used.
Conclusion
In a heavily pre-treated population of patients with R/R HL, the infusion of autologous CD30.CAR-Ts after flu-based lymphodepletion was well tolerated, with a high rate of durable responses. These promising results highlight the feasibility of CAR T-cell therapies in HL, providing a potential new therapeutic option that could be further investigated for its use in both later and earlier stages of the disease.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

