All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ASH 2016 | Obinutuzumab plus Bendamustine Followed by Obinutuzumab Maintenance Prolongs Overall Survival Compared with Bendamustine Alone in Patients with Rituximab-Refractory Indolent Non-Hodgkin Lymphoma: Updated Results of the GADOLIN Study
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, and on December 3rd Professor Bruce D. Cheson, from Georgetown University Hospital, Washington, DC, and colleagues presented an update to the GADOLIN study. The GADOLIN study (NCT01059630) aims to compare obinutuzumab plus bendamustine followed by obinutuzumab maintenance (G-B arm) with bendamustine alone (B arm) in terms of efficacy in an open-label, randomized Phase-III trial in rituximab-refractory indolent non-Hodgkin Lymphoma patients. The newly updated data presented during the congress was including a later cut-off date (April 1st 2016).
Highlights:
- 413 iNHL pts: 204 in G-B arm, 209 in B arm. 335 of iNHL pts had FL: 164 in G-B arm, 171 in B arm.
- Most patients across both arms were refractory to most recent treatment (>92%), having received at least 2 prior therapies (>77%)
- 8 months median follow-up:
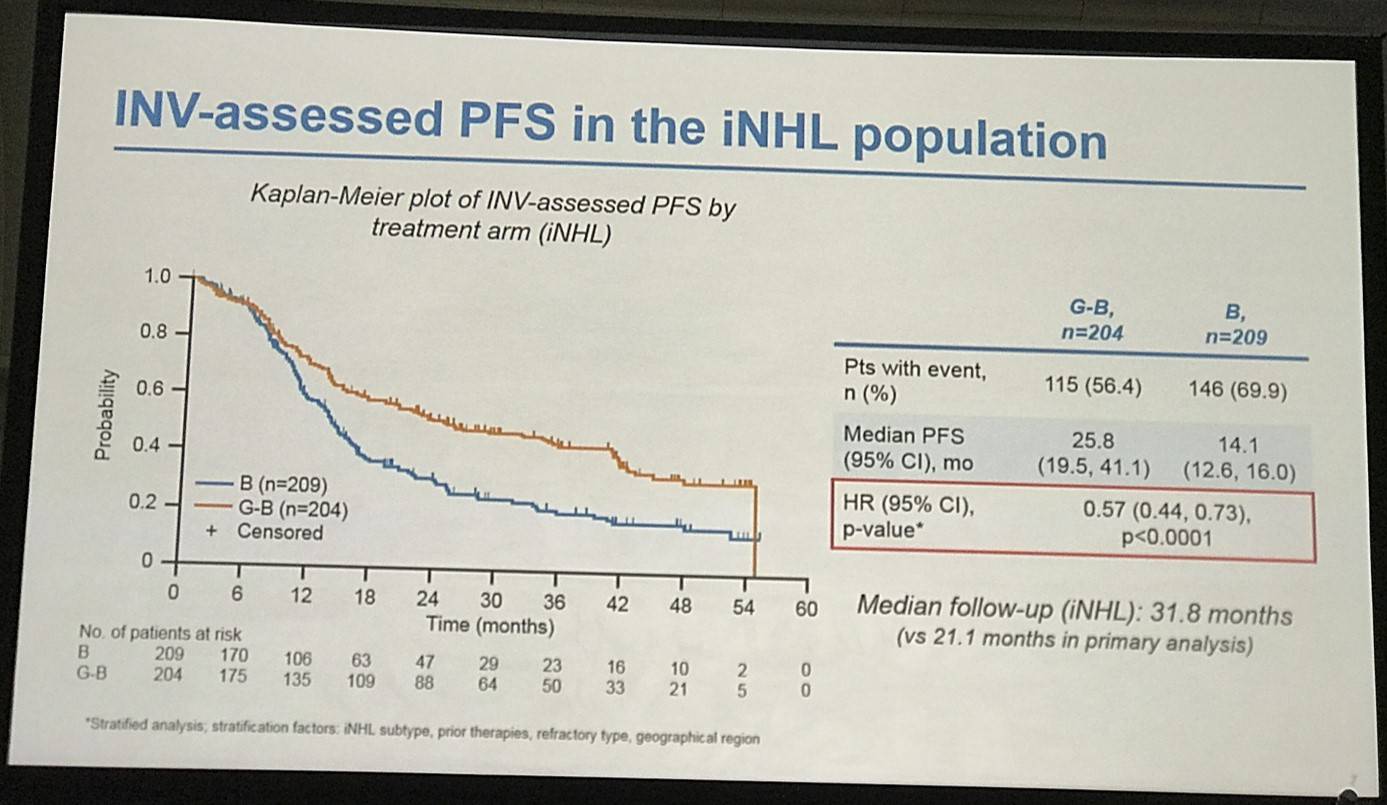
- G-B PFS = 25.8 months, B PFS = 14.0 months, HR = 0.57 (P<0.0001)
- Risk of death or progression reduced by 43% in G-B vs. B arm
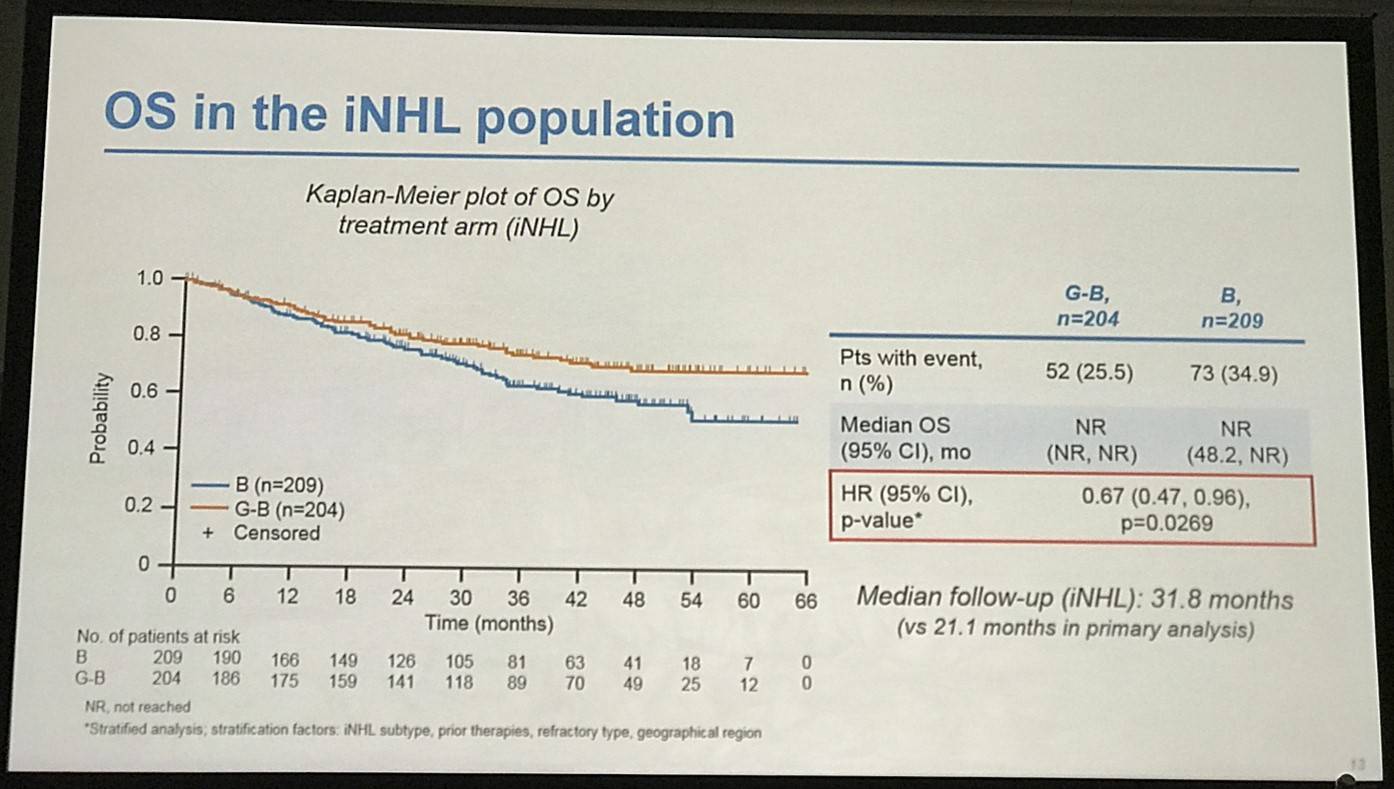
- G-B deaths = 25.5% vs. B deaths = 34.9%. OS HR = 0.67 (P=0.0269)
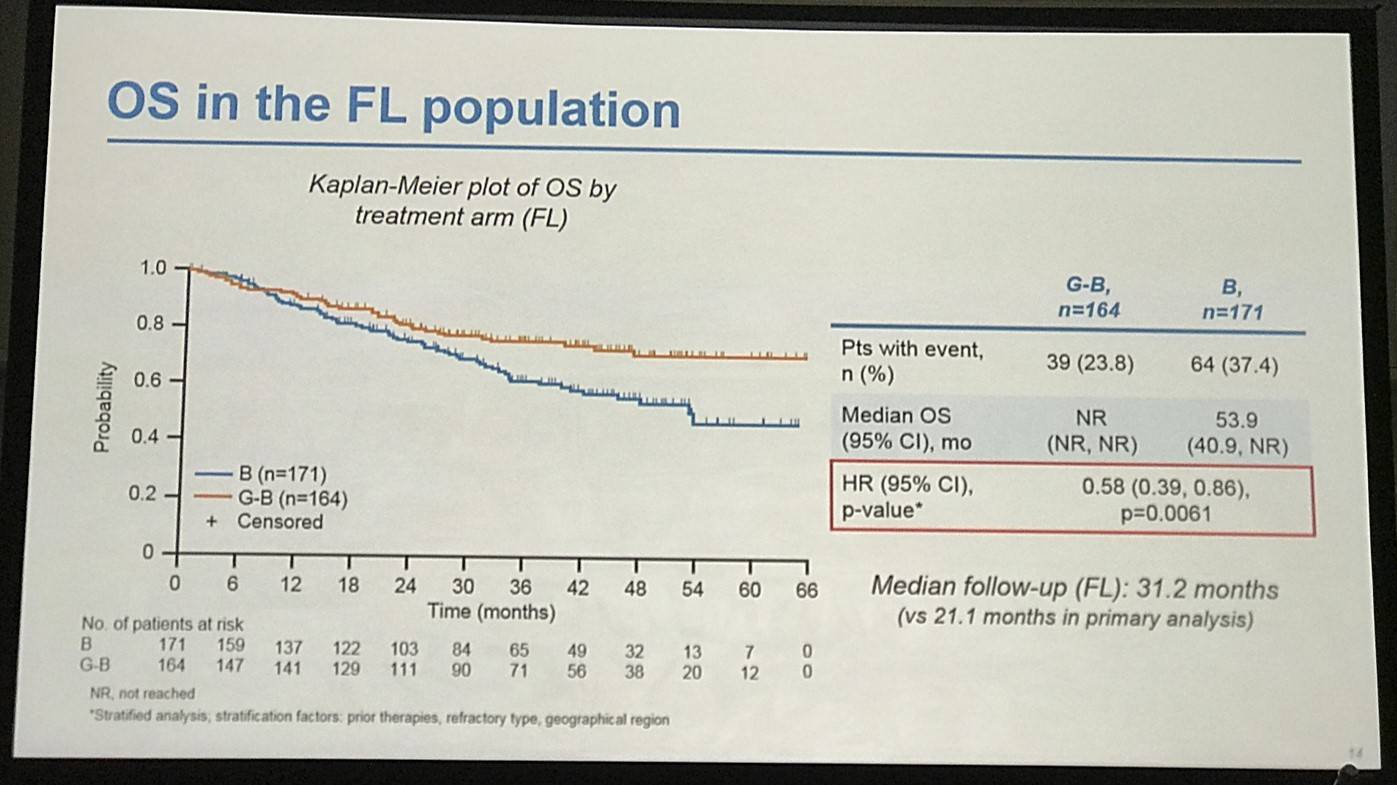
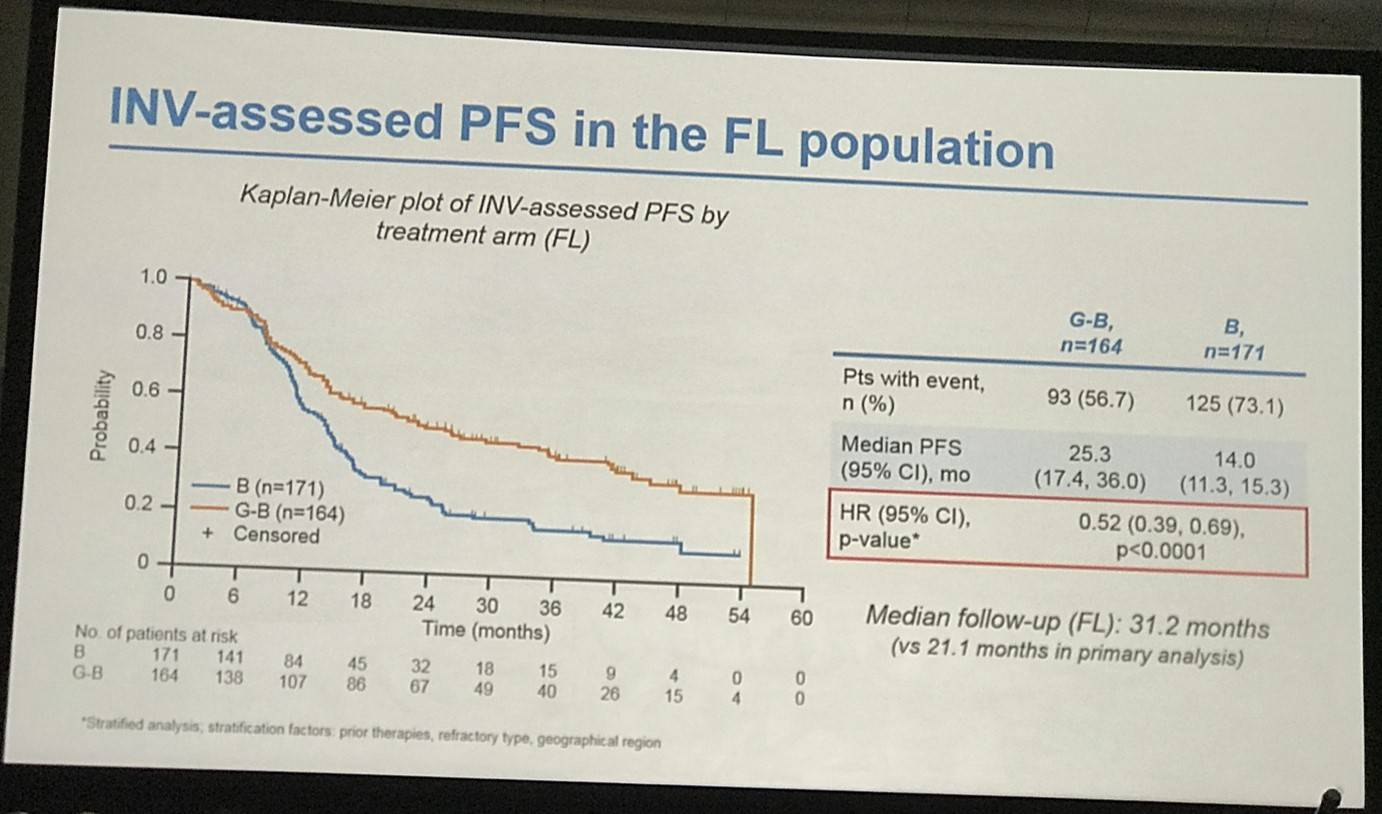
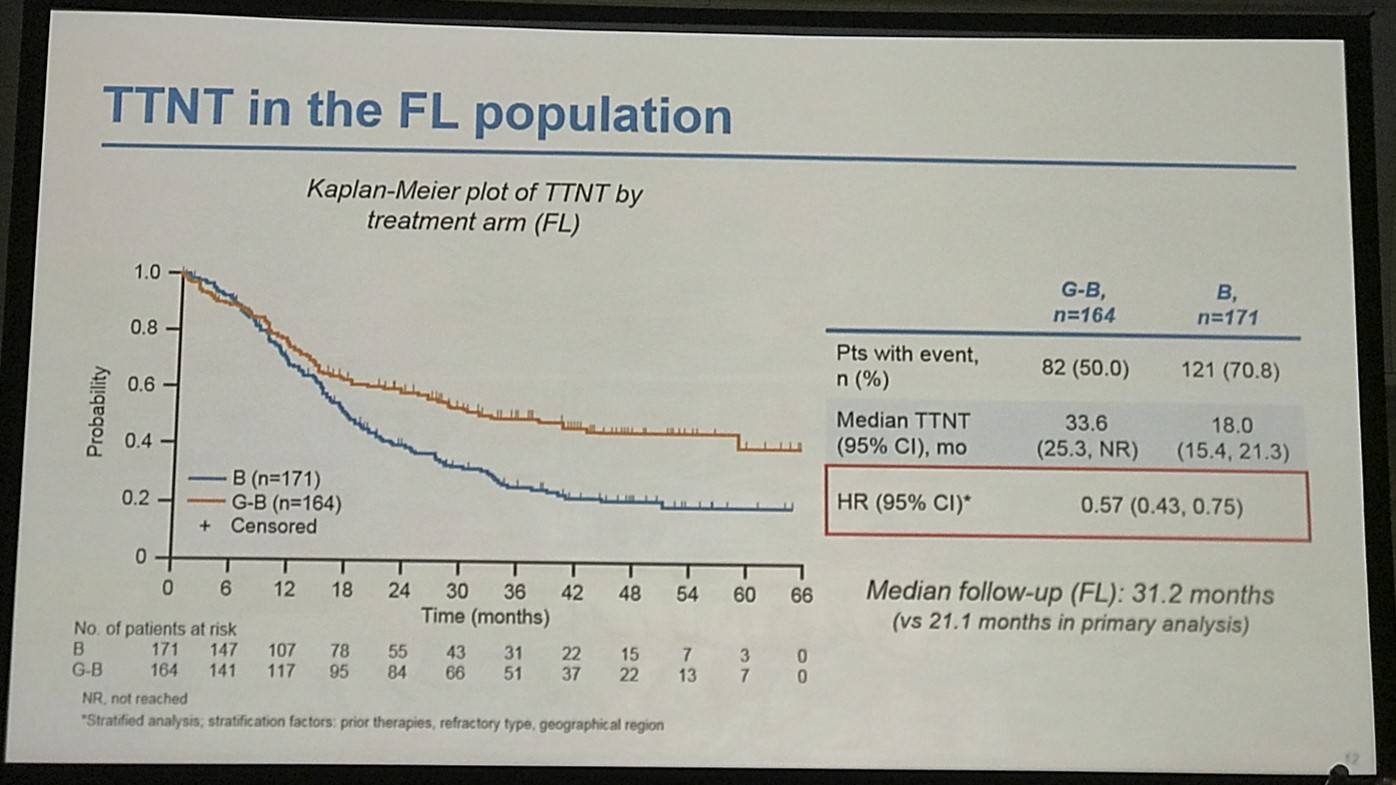
- FL pts results consistent with all iNHL pts results
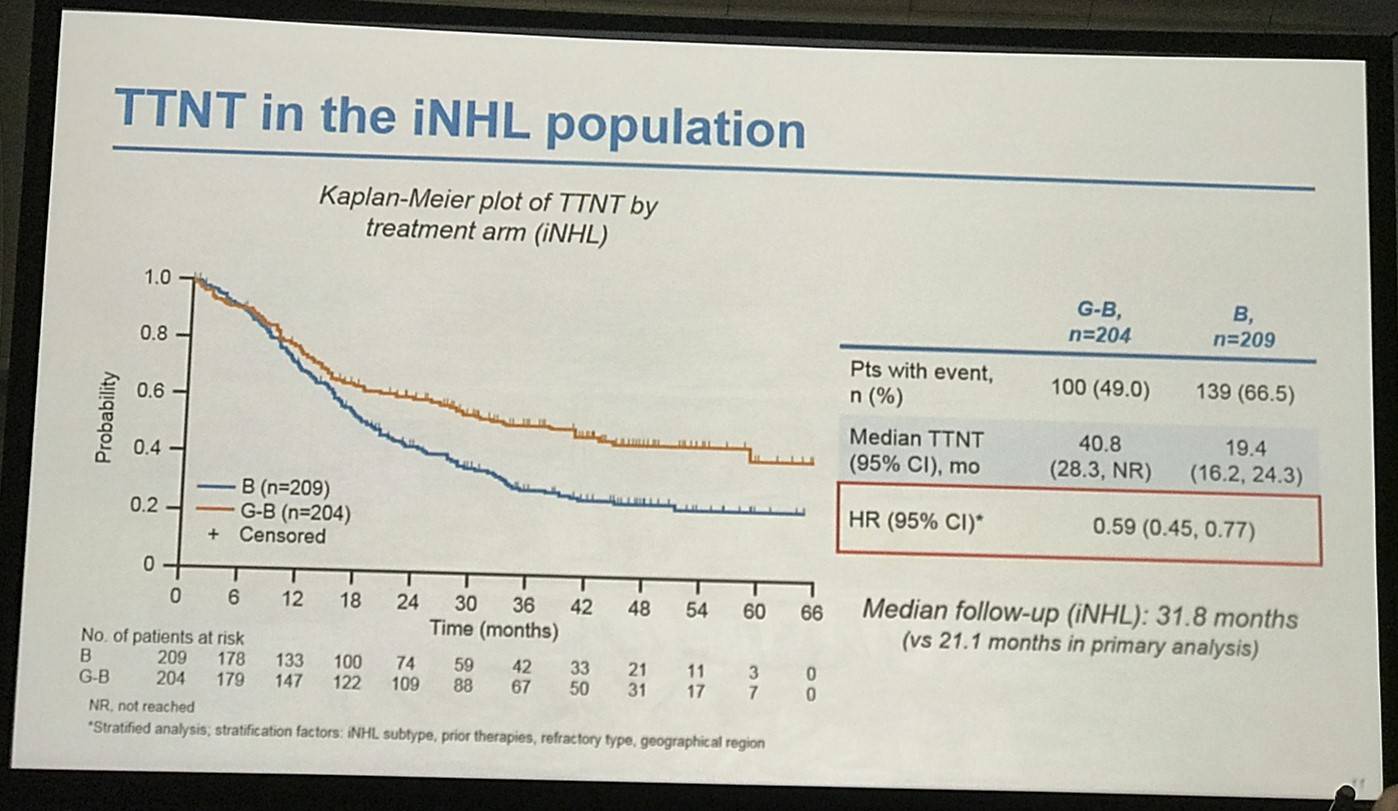
- Median TTNT G-B = 40.8 months vs. B = 19.4 months
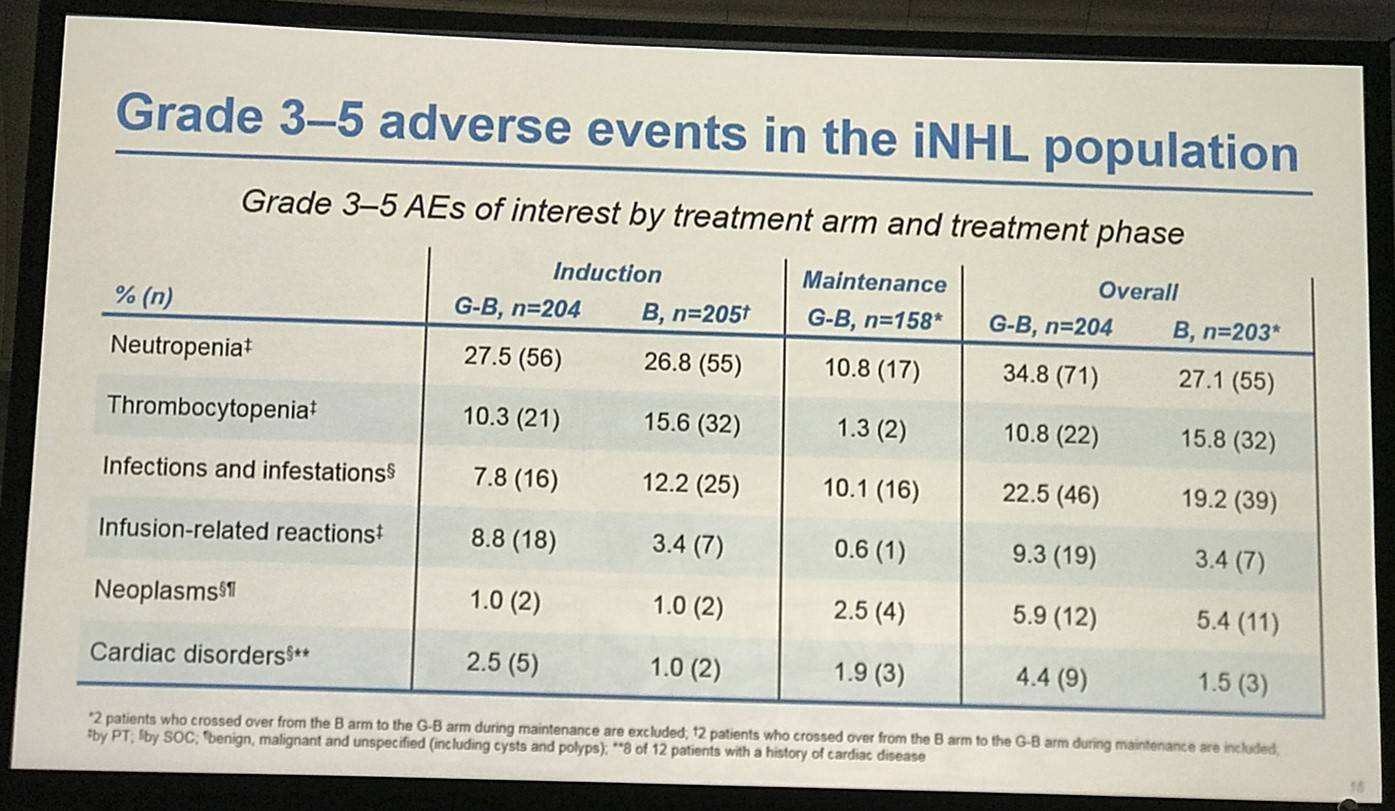
- G-B had higher incidence of ≥ grade 3 AEs (72.5%) than B (65.5%)
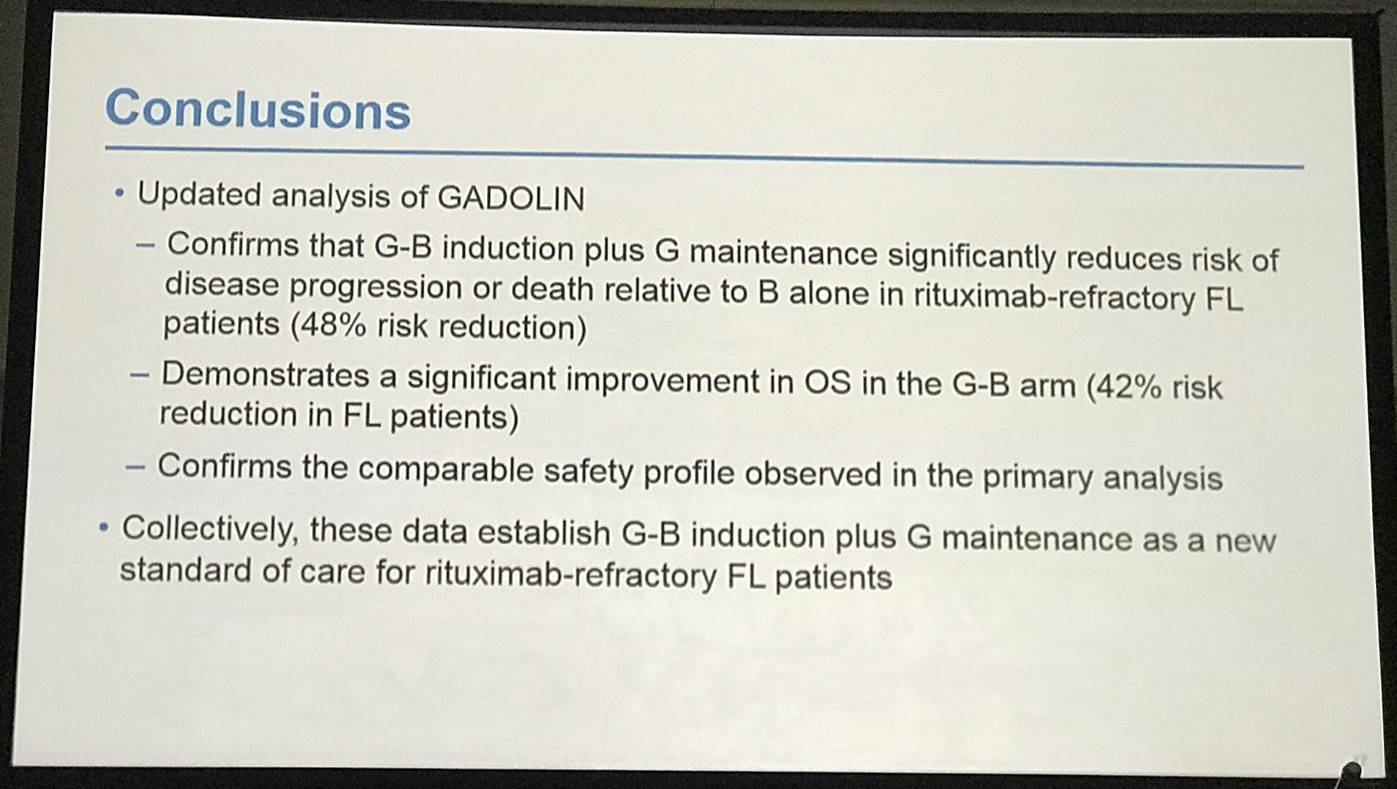
Professor Bruce D. Cheson concluded his talk by stating that the updated analysis with a 10 month longer follow-up window is consistent with the originally reported increase in PFS and OS of G-B vs. B in iNHL patients.
Abstract:
Background: Treatment options for patients (pts) with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL) are limited. GADOLIN (NCT01059630) is an open-label, randomized, Phase 3 trial comparing the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) plus bendamustine (B) induction, followed by G maintenance (G-B arm), with B induction (standard of care) in rituximab-refractory iNHL pts. In the primary analysis, which involved all pts enrolled as of September 1, 2014 (n=396; median observation time, 21.0 months [mo]), median Independent Review Committee (IRC)-assessed progression-free survival (PFS; primary endpoint) was longer in the G-B arm (194 pts; median not reached) than in the B arm (202 pts; 14.9 mo), with a 45% reduction in risk of progression or death (HR 0.55; 95% CI 0.40, 0.74; p=0.0001). Investigator (INV)-assessed PFS was also significantly longer in the G-B arm, but overall survival (OS) data were immature. Safety profiles were comparable. The most common grade ≥3 adverse events (AEs) were neutropenia, thrombocytopenia, anemia, and infusion-related reactions (IRRs). Seventeen additional pts were enrolled after the data cut-off for the primary analysis. Here, we report updated time-to-event and safety results from a planned analysis of all pts (n=413) using a data cut-off of April 1, 2016.
Methods: Enrolled pts were aged ≥18 years (yrs) with documented rituximab-refractory iNHL and an ECOG performance status of 0–2. Pts received either G 1000mg i.v. (days [D] 1, 8, and 15 of cycle [C] 1, and D1 of C2–6) plus B 90mg/m2/day i.v. (D1 and 2 of C1–6), or B monotherapy (120mg/m2/day i.v., D1 and 2 of C1–6); each cycle was 28 days. Following induction, pts in the G-B arm without evidence of progression received G maintenance (1000mg i.v. every 2 mo for 2 yrs or until disease progression, whichever occurred first). In the current analysis, assessments included INV-assessed PFS, OS, time to new anti-lymphoma treatment (TTNT), and safety. Efficacy assessment was performed on the intent-to-treat (ITT) population. Safety analysis included all pts who received any study treatment, excluding 2 pts who crossed over to G-B during maintenance.
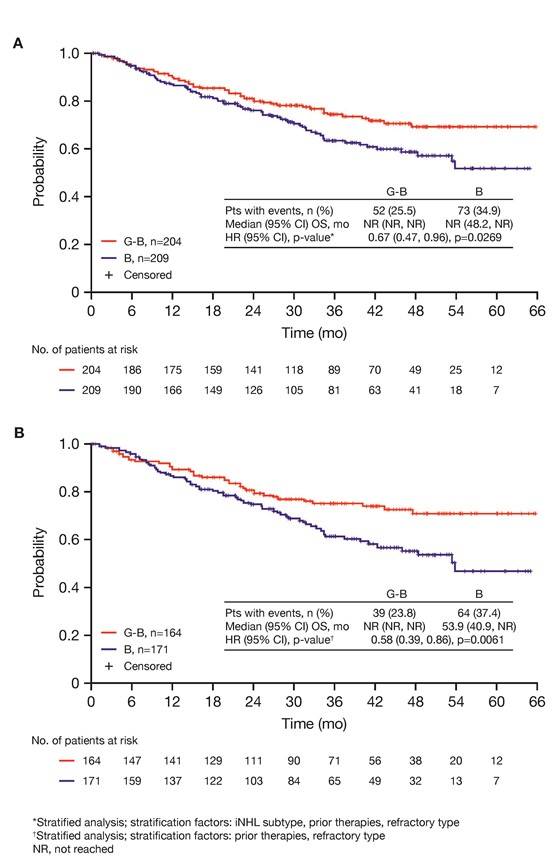
Results: Of 413 iNHL pts in the ITT population (G-B, 204; B, 209), 335 (G-B, 164; B, 171) had FL. Baseline characteristics of the ITT population were balanced between arms. Median number of prior regimens was 2 in both arms (pts with ≤2 prior regimens: G-B, 80.4%; B, 77.5%). Most pts were refractory to their last regimen (G-B, 92.2%; B, 92.3%). After 31.8 mo median follow-up, median INV-assessed PFS was 25.8 mo in the G-B arm and 14.1 mo in the B arm; HR was 0.57 (95% CI 0.44, 0.73; p<0.0001), i.e. a 43% reduction in risk of progression or death for G-B relative to B. Fewer pts died in the G-B arm (25.5%) than the B arm (34.9%), with a HR for OS of 0.67 (95% CI 0.47, 0.96; p=0.0269; risk reduction, 33%; Figure 1A); median OS was not reached for either arm. Results for FL pts were consistent with those for ITT pts (median PFS: 25.3 vs. 14.0 mo [HR 0.52; 95% CI 0.39, 0.69; p<0.0001]; median OS: not reached vs. 53.9 mo [HR 0.58; 95% CI 0.39, 0.86; p=0.0061; Figure 1B]). Median TTNT was also longer in the G-B arm than in the B arm (ITT pts: 40.8 vs. 19.4 mo, respectively [HR 0.59; 95% CI 0.45, 0.77]; FL pts: 33.6 vs. 18.0 mo, respectively [HR 0.57; 95% CI 0.43, 0.75]). The overall safety profile of G-B remained consistent with results of the primary analysis. In the ITT population, there were more grade ≥3 AEs with G-B than with B (72.5% vs. 65.5%, respectively), notably neutropenia (34.8% vs. 27.1%) and IRRs (9.3% vs. 3.5%); grade ≥3 thrombocytopenia (10.8% vs. 15.8%) and anemia (7.4% vs. 10.8%) were less frequent in the G-B arm, while grade ≥3 infections (22.5 % vs. 19.2%) and secondary malignancies (5.9% vs. 5.4%) were reported with a similar incidence. Serious AEs were more frequent in the G-B arm (43.6% vs. 36.9%), but the incidence of grade 5 (fatal) AEs was similar (7.8% vs. 6.4%). Safety results in FL pts were comparable with those in all iNHL pts.
Conclusions: Updated analysis of the GADOLIN study with ~10 mo additional follow-up confirms the previously reported PFS benefit of G-B over B in pts with rituximab-refractory iNHL, and demonstrates a significant improvement in OS in the G-B arm. No new safety signals were detected.
Figure 1. OS results: A) ITT population; B) FL pts
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

