All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Bispecific antibodies for patients with R/R B-cell lymphoma: Latest updates from phase II trials
Bispecific antibodies that bind CD20 on B cells and CD3 on T cells are a promising treatment prospect for B-cell non-Hodgkin lymphoma (NHL). The agents currently under investigation include glofitamab, epcoritamab, odronextamab, and mozunetuzumab.
Updated data from the phase II trials of glofitamab and epcoritamab in patients with relapsed/refractory (R/R) NHL were presented during the European Hematology Association (EHA) 2022 Congress. Late-breaking safety and efficacy data for epcoritamab (EPCORE NHL-1 study; NCT03625037) was presented by Catherine Thieblemont,1 and the latest phase II data for glofitamab (NCT03075696) was presented by Michael Dickinson.2 Below, we summarize the results.
Epcoritamab1
Study design
This is a phase II dose expansion trial of subcutaneous epcoritamab in patients with R/R large B-cell lymphoma (LBCL), high-grade B-cell lymphoma, transformed follicular lymphoma, or primary mediastinal B-cell lymphoma who had received ≥2 prior lines of antineoplastic therapy; had an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–2; and fluorodeoxyglucose-positron emission tomography (PET) avid and measurable disease by computer tomography (CT) or magnetic resonance imaging. Patients received priming and intermediate doses of epcoritamab followed by full doses of epcoritamab (48 mg) as 1 mL subcutaneous injections for 28 cycles.
The primary endpoint of overall response rate (ORR) was assessed by an independent review committee (IRC) based on Lugano classification and assessed by PET-CT. Secondary endpoints included duration of response (DOR), time to respond, progression-free survival (PFS), overall survival (OS), complete remission (CR) rate, safety, and tolerability.
Results
At the data cutoff of January 31, 2022, a total of 157 patients were included with a median age of 64 years (range, 20–83 years) and a median of 3 prior lines of therapy (range, 2–11 lines of therapy), 89% of these patients had diffuse LBCL (DLBCL).
Efficacy
At a median follow-up of 10.7 months, IRC assessed ORR was 63% and CR rate was 39% (Table 1). The median duration of response was 12 months in all patients and not reached in patients achieving CR, with 89% of patients remaining in CR at 9 months. The ORR was similar across subgroups of age, prior lines of therapy, and de novo or transformed disease (Figure 1). The median PFS was of 4.4 months (range, 3–7.9 months) and 43.9% of patients showed PFS at 6 months. The median OS has not yet been reached and was 70.6% and 56.9% months at 6 and 12 months, respectively. Exploratory circulating tumor DNA analysis showed that durable responses and PFS were associated with minimal residual disease negativity in 45.8% of patients.
Table 1. Response rates*
|
CI, confidence interval; CR, complete response; IRC, independent review committee; LBCL, large B-cell lymphoma; NR, not reached; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease. |
|
|
Best overall response by IRC, % (unless |
Total patients with LBCL |
|---|---|
|
ORR (95%CI) |
63 (55–71) |
|
CR (95% CI) |
39 (31–47) |
|
PR |
24 |
|
SD |
3 |
|
PD |
24 |
|
Median time to response (range), months |
1.4 (1.0–8.4) |
|
Median time to CR (range), months |
2.7 (1.2–11.1) |
|
Median OS and PFS for complete responders |
NR |
Figure 1. Response rates in subgroups*
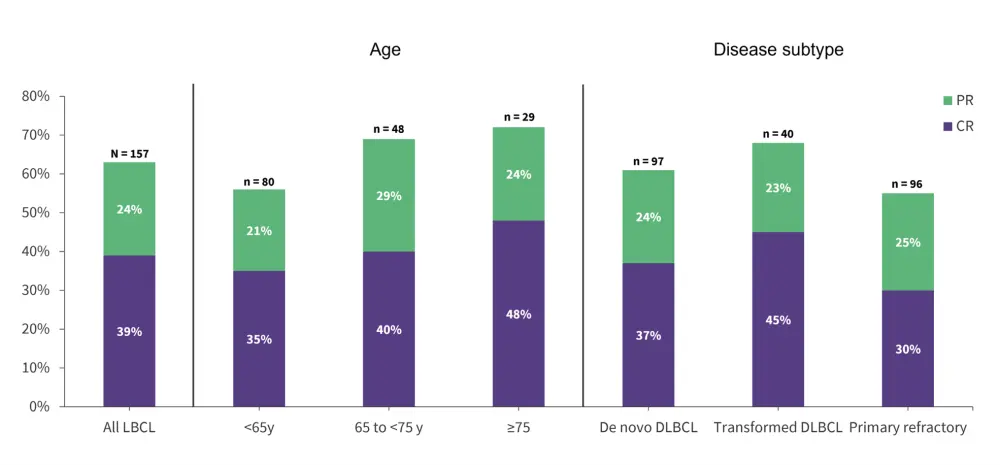
CR, complete response; CAR, chimeric antigen receptor; DLBCL, diffuse large B-cell lymphoma; LBCL, large B-cell lymphoma; PR, partial response.
*Adapted from C Thieblemont.1
Safety
The most common treatment-emergent adverse events (TEAEs) of any grade were cytokine release syndrome (CRS) (49.6%), neutropenia (28%), pyrexia (23.5%), fatigue (22.9%), diarrhea (20.4%), nausea (19.8%), injection site reaction (19.8%) and anemia (17.9%; Figure 2). Most adverse events (AEs) were low grade and occurred early in treatment Cycle (C) 1–3. The incidence of CRS events was 31.8% (Grade 1), 15.3% (Grade 2), and 2.5% (Grade 3); occurring mainly after the first full dose on C1, Day (D) 15 (42.8%).
Ten patients experienced immune effector cell-associated neurotoxicity syndrome (ICANS) of which 9 were Grade 1–2 and resolved and only one patient had Grade 5 ICANS. Among the patients discontinuing treatment, 53% discontinued due to progressive disease and 7% due to AEs, while 32% of patients remained on treatment.
Figure 2. TEAEs ≥15% by grade*
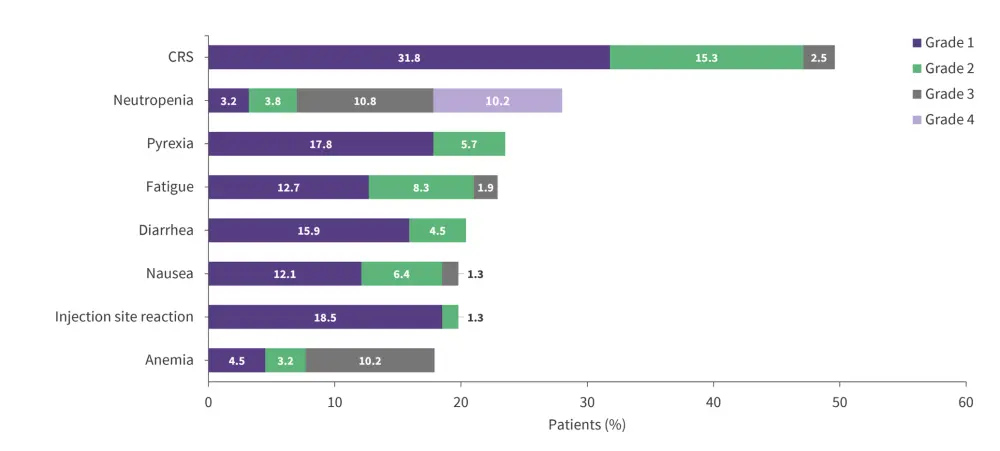
CRS, cytokine release syndrome; TEAEs, treatment-emergent adverse events.
*Adapted from Thieblemont.1
Glofitamab2
Study design
This is a phase II dose expansion trial of glofitamab in patients with R/R DLBCL, high-grade B-cell lymphoma, transformed follicular lymphoma, or primary mediastinal B-cell lymphoma who received ≥2 prior therapies and had an ECOG Performance Status of 0–1. Patients received pretreatment CRS mitigation with a single dose of intravenous 1000 mg obinutuzumab and glofitamab 30 mg on D1 of C2–12.
The primary endpoint was CR rate assessed by IRC. Secondary endpoints included ORR, DOR, duration of CR (doCR), PFS and OS.
Results
At the data cutoff of March 14, 2022, a total of 154 heavily pre-treated and highly refractory patients with a median age of 66 years were included, 71% of which had DLBCL and a median number of 3 prior lines of therapy (range, 2–7 lines of therapy).
Efficacy
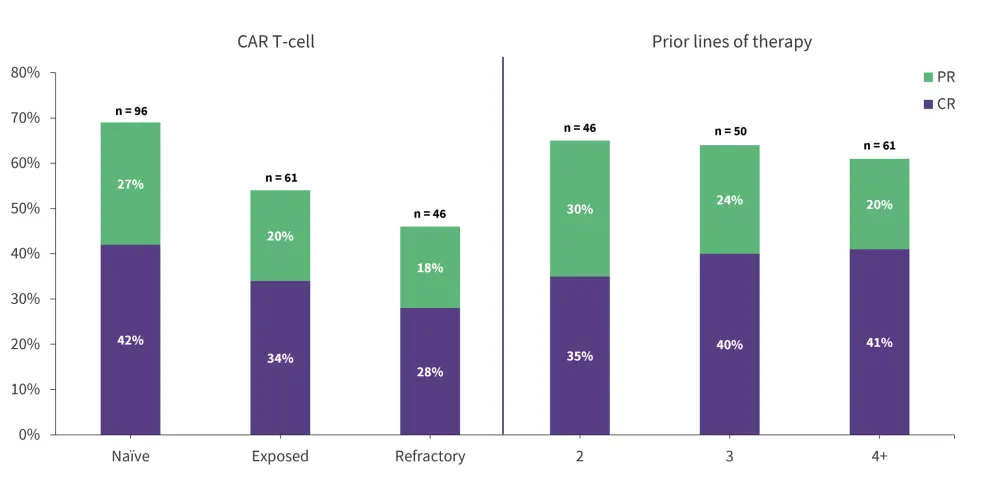
At a median follow-up of 12.6 months, a CR rate and ORR of 39.4% and 51.6%, respectively, were achieved. The median time to first CR was 42 days and most responses were achieved early during treatment. The CR rate was significantly greater in the primary efficacy population compared with the historical cohort (35.2% vs 20%; p < 0.0001). Responses were consistent across selected subgroups such as age, disease subtype, prior CAR T-cell therapy and R/R to last prior therapy (Figure 3).
Figure 3. Complete response rates in subgroups*
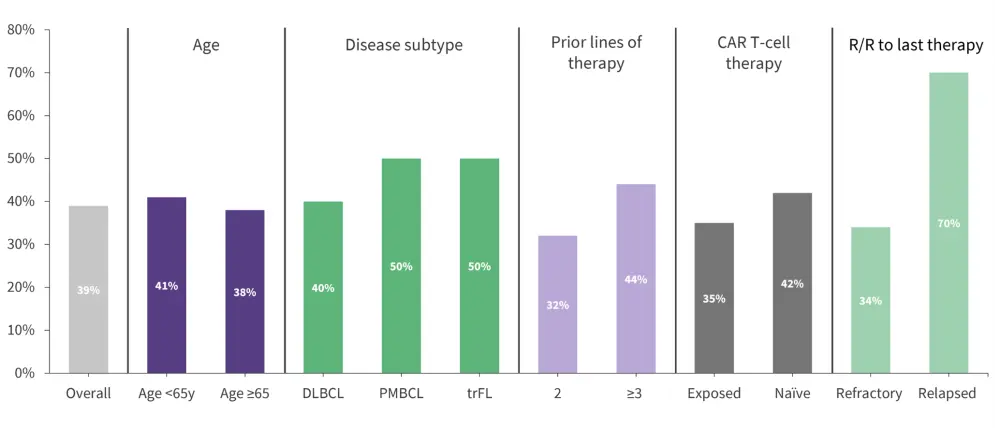
CAR, chimeric antigen receptor; DLBCL, diffuse large B-cell lymphoma; HGBCL, high-grade B-cell lymphoma; PMBCL, primary mediastinal B-cell lymphoma; trFL, transformed follicular lymphoma.
*Adapted from Dickinson.2
Both the median DOR and DoCR was 10.6 months. The estimated 12 months DOR and DoCR were observed in 63.6% and 77.6% of patients and the OR and CR were ongoing in 66.3% and 80.3% of patients at the data cutoff, respectively.
DoCR was consistent with a supporting cohort of patients from the phase I trial, eligible for dose expansion and treated with glofitamab doses ≥10 mg and <30 mg, with a median follow-up of 24.8 months and a median DoCR of 34.2 months. Ongoing CR was observed in 62.9% of patients.
The median PFS and OS were 4.9 and 11.5 months, and the event-free survival and OS rate at 12 months was 37.1% and 49.8%, respectively.
Safety
AEs occurred in 98.7% of patients but were mostly low-grade. The most common TEAEs of any grade included CRS, neutropenia, anemia, and pyrexia (Figure 4). The incidence of TEAEs leading to treatment discontinuation was low (3.2%).
Figure 4. AEs ≥15% by grade and TEAEs*
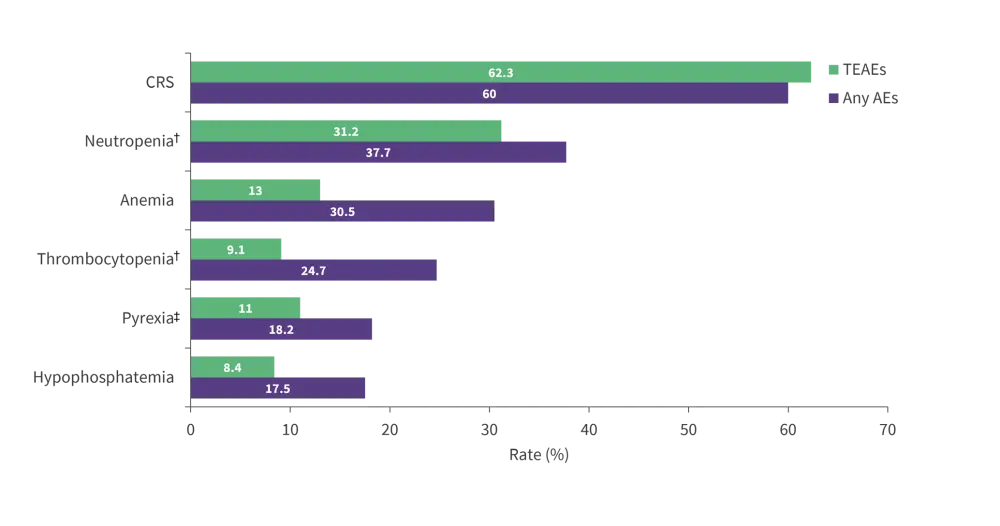
AEs, adverse events; CRS, cytokine release syndrome; TEAEs, treatment-emergent adverse events.
*Adapted from Dickinson.2
The incidence of CRS events was 47.4% (Grade 1) and the median time to CRS onset from C1, D8 was 13.6 months (Table 2). The incidence of CRS by cycle was 54.5% (C1, D8–14), 30.4% (C1, D15–21), 26.8% (C2), 0.9% (C3), and 2.0% (C4+) with most events being low grade. Pretreatment with dexamethasone reduced the occurrence of overall CRS compared to pretreatment with any corticosteroid (47.5% vs 68.4%, respectively) and showed no ≥2 grade CRS with 10 mg or 30 mg of glofitamab. Infections of any grade occurred in 38.3% of patients and ICANS occurred in 7.8% of patients.
Table 2. AEs and TEAEs*
|
AEs, adverse events; C, cycle; D, day; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; SAEs, serious adverse events; SA TEAEs, serious treatment-emergent adverse events; TEAEs, treatment-emergent adverse events. |
|
|
AEs and TEAEs, % (unless otherwise stated) |
Total (N = 154) |
|---|---|
|
AE |
98.7 |
|
Grade 3–4 AEs |
56.5 |
|
SAEs |
47.4 |
|
Grade 5 fatal AE† |
5.2 |
|
AE leading to treatment discontinuation |
9.1 |
|
TEAEs |
90.9 |
|
Grade 3–4 TEAEs |
41.6 |
|
TE SAEs |
29.9 |
|
Grade 5 fatal TEAE |
0 |
|
AE leading to treatment discontinuation |
3.2 |
|
CRS (any grade)‡ |
63.0 |
|
Grade 1 |
47.4 |
|
Grade 2 |
11.7 |
|
Grade 3 |
2.6 |
|
Grade 4 |
1.3 |
|
Median time to CRS onset from C1, D8 dose (range), hours |
13.6 |
|
Infections (all grades) |
38.3 |
|
Grade ≥3 |
14.9 |
|
Neutropenia§ (all grades) |
37.7 |
|
Grade ≥3 |
26.6 |
|
Febrile neutropenia (all grades) |
2.6 |
|
Grade ≥3 |
2.6 |
|
Neurologic AEs‖ (all grades) |
38.3 |
|
Grade ≥ 3 |
3.2 |
|
ICANS¶ (derived) |
|
|
All grades |
7.8 |
|
Grade ≥ 3 |
2.6 |
Conclusion
The two ongoing phase II trials showed that both epcoritamab and glofitamab achieved high CR rates in highly refractory patients with B-cell lymphoma. The responses remained consistent across the key subgroups of age, disease subtype, CAR T-cell therapy, and prior lines of therapy. Both drugs were well tolerated with a low rate of treatment discontinuation. The occurrence of CRS was as expected, mostly low grade and occurring early during treatment. The findings indicate that epcoritamab and glofitamab lead to deep and durable responses in a challenging-to-treat population of highly refractory patients with R/R B-cell lymphoma.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

